PubMed Central, CAS, DOAJ, KCI

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Volume 40(1); 2023 > Article
-
Original article
The effect and therapeutic compliance of adjuvant therapy in patients with cholangiocarcinoma after R0 resection: a retrospective study -
Han Taek Jeong
 , Joonkee Lee
, Joonkee Lee , Hyeong Ho Jo
, Hyeong Ho Jo , Ho Gak Kim
, Ho Gak Kim , Jimin Han
, Jimin Han
-
Journal of Yeungnam Medical Science 2023;40(1):65-77.
DOI: https://doi.org/10.12701/jyms.2022.00213
Published online: May 26, 2022
Department of Internal Medicine, Daegu Catholic University School of Medicine, Daegu, Korea
- Correspondence author: Jimin Han, MD, PhD Department of Internal Medicine, Daegu Catholic University School of Medicine, 33 Duryugongwon-ro 17-gil, Nam-gu, Daegu 42472, Korea Tel: +82-53-650-3442 • Fax: +82-53-621-4487 • E-mail: jmhan@cu.ac.kr
Copyright © 2023 Yeungnam University College of Medicine, Yeungnam University Institute of Medical Science
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- This study aimed to compare clinical outcomes between surveillance and adjuvant therapy (AT) groups after R0 resection for cholangiocarcinoma (CCA).
-
Methods
- A total of 154 patients who underwent R0 resection for CCA at the Daegu Catholic University Medical Center between January 2010 and December 2019 were included. Overall survival (OS) and progression-free survival (PFS) were analyzed.
-
Results
- The median follow-up duration was 899 days. There were 109 patients in the AT group and 45 patients in the surveillance group. The patients in the AT group were younger (67 years vs. 74 years, p<0.001) and included more males (64.2% vs. 46.7%, p=0.044). The proportion of patients with stage III CCA was larger in the AT group than in the surveillance group (13.8% vs. 2.2%, p=0.005). In addition, AT did not improve OS (5-year OS rate, 69.3% in the AT group vs. 64.2% in the surveillance group, p=0.806) or PFS (5-year PFS rate, 42.6% in the AT group vs. 48.9% in the surveillance group, p=0.113). In multivariate analysis using the Cox proportional hazards model, stage III CCA (hazard ratio [HR], 10.81; 95% confidence interval [CI], 2.92–40.00; p<0.001) was a significant predictor of OS. American Society of Anesthesiologists classification II (HR, 0.50; 95% CI, 0.31–0.81; p=0.005), and American Joint Committee on Cancer stages II (HR, 3.14; 95% CI, 1.25–7.89; p=0.015) and III (HR, 8.08; 95% CI, 2.80–23.32; p<0.001) were independent predictors of PFS.
-
Conclusion
- AT after R0 resection for CCA did not improve OS or PFS.
- Cholangiocarcinoma (CCA) is an epithelial cell malignancy that occurs anywhere in the biliary system [1]. It is classified according to the anatomical site of origin as intrahepatic, perihilar, or distal CCA [1,2]. The incidence of CCA varies globally, ranging from 2.3 to14.5 per 100,000 people in the East and 0.3 to 3.4 per 100,000 people in the West [2]. Despite advances in treatment, the 5-year overall survival rate (OSR) remains poor, ranging from 10% to 40% [3,4]. In Korea from 2006 to 2015, the incidence of CCA decreased but was still high at 7.8 per 100,000 people for intrahepatic CCA and 6.7 per 100,000 people for extrahepatic CCA [5]. The 5-year OSRs of extrahepatic and intrahepatic CCA in Korea were 27.8% and 15.9%, respectively [5].
- Although surgical resection is the only curative treatment, most patients are diagnosed with advanced disease, and only 20% to 30% of cases are resectable at diagnosis [3,4]. Moreover, more than two-thirds of patients relapse within 5 years after surgery [3]. Therefore, various studies on adjuvant therapy (AT) for CCA have investigated many chemotherapeutic agents, radiotherapy, or both, which had previously been demonstrated to be effective for locally advanced and metastatic CCA [3]. However, the few large prospective studies on AT conducted to date have produced disappointing results [6-10]. The effect of AT on CCA has been inconsistent among several recent retrospective studies [11-20]. In particular, if patients have undergone R0 resection, the National Comprehensive Cancer Network guidelines recommend all possible options: observation, systemic therapy, or clinical trial [21].
- Therefore, this study aimed to retrospectively investigate the effect of AT on CCA after R0 resection. We also analyzed prognostic factors associated with overall survival (OS) and progression-free survival (PFS). Furthermore, the therapeutic compliance of AT was evaluated.
Introduction
- Ethical statements: This study was performed in compliance with the ethical guidelines of the revised Helsinki Declaration of 2013. This study was reviewed and approved by the Institutional Review Board (IRB) of the Daegu Catholic University Medical Center (IRB No: CR-21-107-L). Since this study was retrospective, the need for informed consent was waived.
- 1. Study population
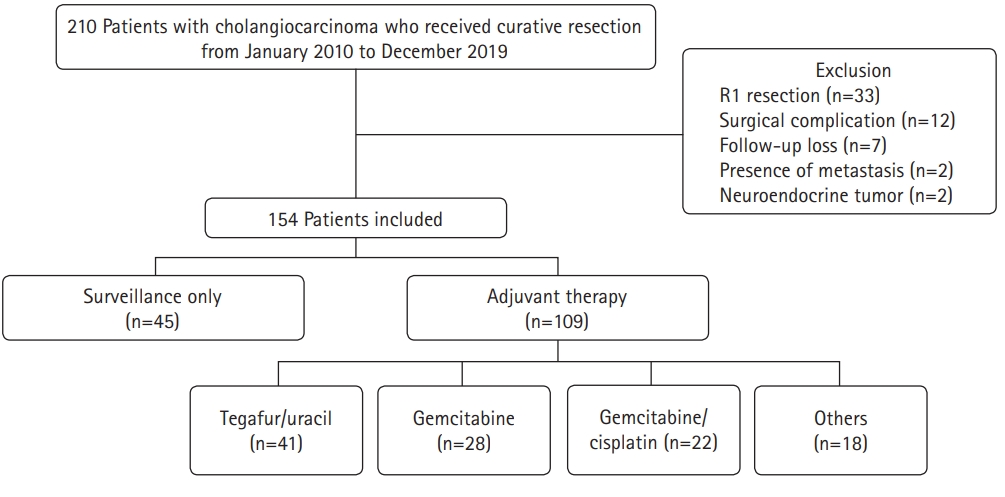
- A total of 210 patients who underwent curative surgery for CCA at Daegu Catholic University Medical Center between January 2010 and December 2019 were eligible for this study (Fig. 1). Cases were collected using diagnostic codes (C221, C240, C248, and C249) based on the 8th revision of the Korean Standard Classification of Diseases. The following were exclusion criteria: (1) positive resection margins, (2) death due to surgical complications, (3) follow-up at another hospital after surgery, (4) distant metastasis at the time of surgery, and (5) neuroendocrine tumors.
- 2. Study design
- This was a retrospective single-center study. The following data were collected from medical records: demographics, preoperative serum carbohydrate antigen 19-9 (CA 19-9) and carcinoembryonic antigen (CEA) levels, tumor location, AT regimen, radiologic findings, and pathologic findings such as perineural invasion, differentiation, lymphovascular invasion, and extent of resection. Survival data were also obtained from the medical records. Disease stage was reclassified based on the American Joint Committee on Cancer (AJCC) Cancer Staging Manual (8th edition). Patients were then divided into surveillance and AT groups to investigate the effects of AT. We also investigated the therapeutic adherence to AT.
- 3. Definitions
- Patients who received planned chemotherapy or chemoradiotherapy after R0 resection were assigned to the AT group. Patients who continued follow-up without adjuvant chemotherapy or chemoradiotherapy after surgery were assigned to the surveillance group. OS was defined as the time from the date of surgery to the date of death from any cause. PFS was defined as the time from the date of surgery to the date of recurrence. The American Society of Anesthesiologists (ASA) classification was used to evaluate patient performance status. The cut-off level for serum CA 19-9 was 37 U/mL, and that for serum CEA was 5.2 ng/mL.
- 4. Treatment strategy and follow-up
- The AT regimen for each patient was decided at the discretion of the clinician. In the AT group, an abdominal computed tomography (CT) scan was performed every 2 or 3 months during the AT and every 6 or 12 months thereafter. In the surveillance group, a CT scan was performed every 6 or 12 months. In both groups, a CT scan was also performed regardless of the follow-up schedule if the clinician determined that it was necessary because of symptoms or signs such as abdominal pain, fever, and jaundice. Follow-up continued until December 31, 2021, or until death.
- 5. Statistical analysis
- Statistical analysis was performed using IBM SPSS ver. 19.0 for Windows (IBM Corp., Armonk, NY, USA). The chi-square or Fisher exact tests were used to compare categorical variables. Since the continuous variables were not normally distributed, they were described as medians with interquartile ranges, and the Mann-Whitney U-test was used to compare them. OS and PFS were analyzed using Kaplan-Meier survival analysis. The OS and PFS of the AT group and surveillance group were compared with the log-rank test. Subgroup analysis of the following variables was performed: lymphovascular invasion, perineural invasion, AJCC stage, and AT regimens. Univariate analysis was used to identify the prognostic factors associated with OS and PFS in patients with R0-resected CCA. Multivariate analysis was performed using the Cox proportional hazards model with backward elimination for the factors that were significant in the univariate analysis or those considered clinically meaningful in previous studies. The results are presented as hazard ratios (HRs) and 95% confidence intervals (CIs). Statistical significance was defined as a p-value of <0.05 (two-tailed).
Methods
- 1. Baseline characteristics of patients
- A total of 154 patients with CCA who underwent R0 resection during the study period were investigated. Of these, 109 patients (70.8%) received AT (AT group) and 45 patients (29.2%) were only followed up (surveillance group). The baseline patient characteristics are shown in Table 1. The patients in the AT group were younger than those in the surveillance group (67 years vs. 74 years, p<0.001). There was also a higher proportion of males and patients with AJCC stage III disease in the AT group than in the surveillance group (64.2% vs. 46.7%, p=0.044 and 13.8% vs. 2.2%, p=0.005, respectively). However, other characteristics, including ASA classification, tumor marker levels, tumor location, and pathologic findings, were not significantly different between the two groups. There were 29 intrahepatic CCA (18.8%), 43 perihilar CCA (27.9%), and 82 distal CCA cases (53.2%). Most cases were adenocarcinomas (96.8%). The most common histologic differentiation was moderately differentiated cancer (56.5%), followed by poorly differentiated (25.3%) and well-differentiated (14.3%) cancer. Lymphovascular invasion and perineural invasion were observed in 53 (34.4%) and 91 patients (48.9%), respectively. The most common AJCC stage was stage II (73.4%), followed by stage I (16.2%) and stage III (10.4%). With regard to the AT regimen, tegafur/uracil (37.6%) was the most commonly used, followed by gemcitabine (25.7%) and gemcitabine/cisplatin (20.2%). Twelve patients in the AT group (11.0%) received chemoradiotherapy.
- 2. Survival analysis
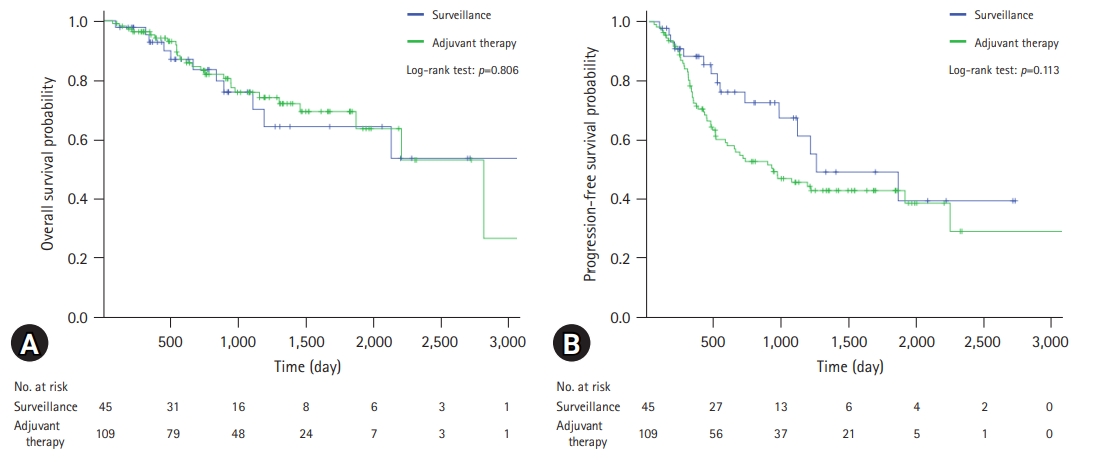
- The survival analysis of the patients is shown in Table 2. The median follow-up duration was 899 days. There was no statistically significant difference between the two groups (924 days in the AT group vs. 788 days in the surveillance group, p=0.404). The 1-year, 3-year, and 5-year OSRs for all patients were 94.5%, 75.6%, and 68.0%, respectively. There were no significant differences between the two groups (95.2%, 75.8%, and 69.3% in the AT group vs. 92.8%, 75.9%, and 64.2% in the surveillance group, respectively, p=0.806). In contrast, the PFS rate (PFSR) was higher in the surveillance group, although the difference was not statistically significant (p=0.113). The 1-year, 3-year, and 5-year PFSRs for the surveillance group were 88.2%, 67.3%, and 48.9%, respectively, and those for the AT group were 70.3%, 45.4%, and 42.6%, respectively.
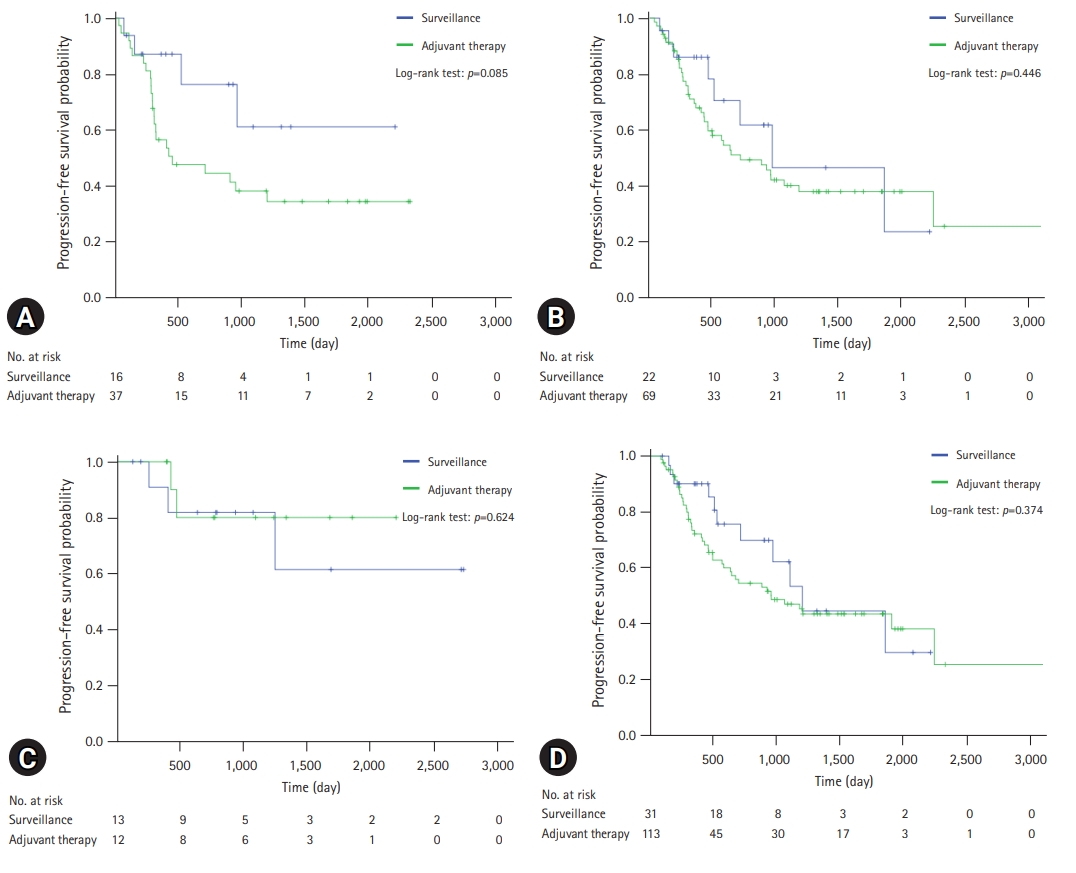
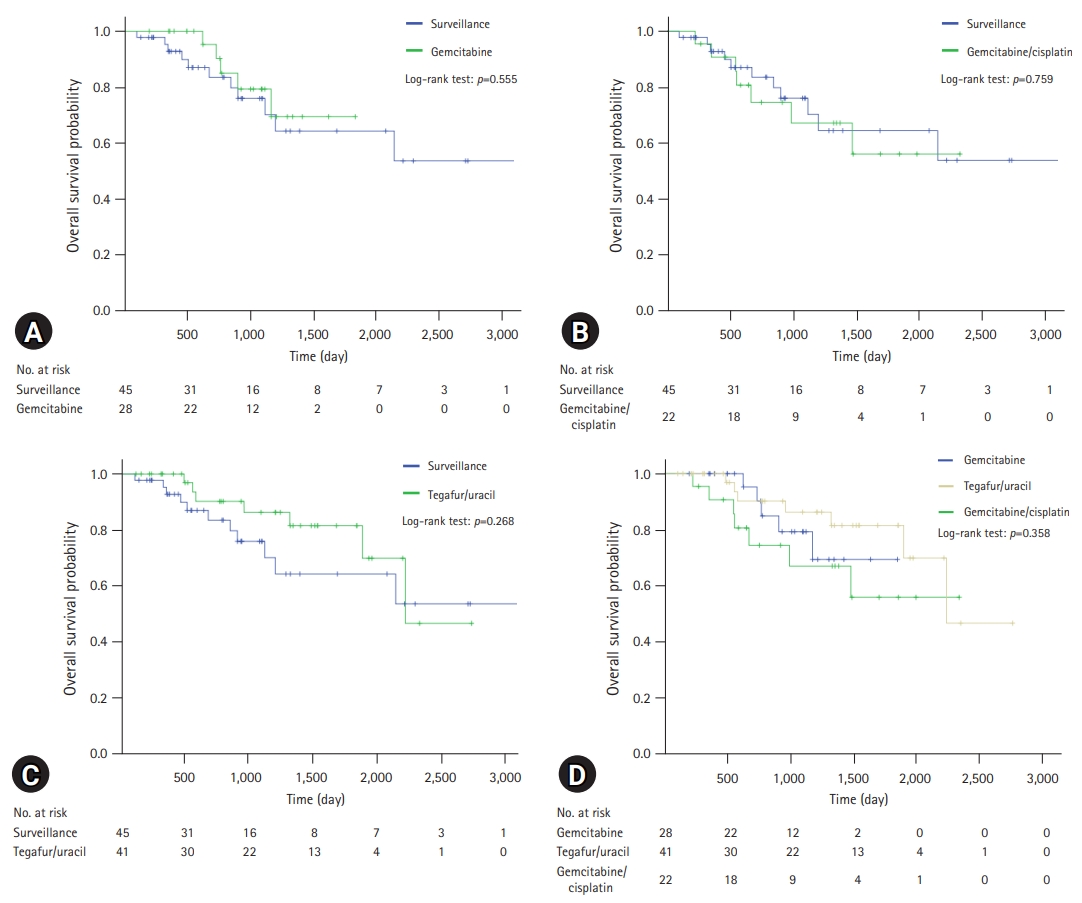
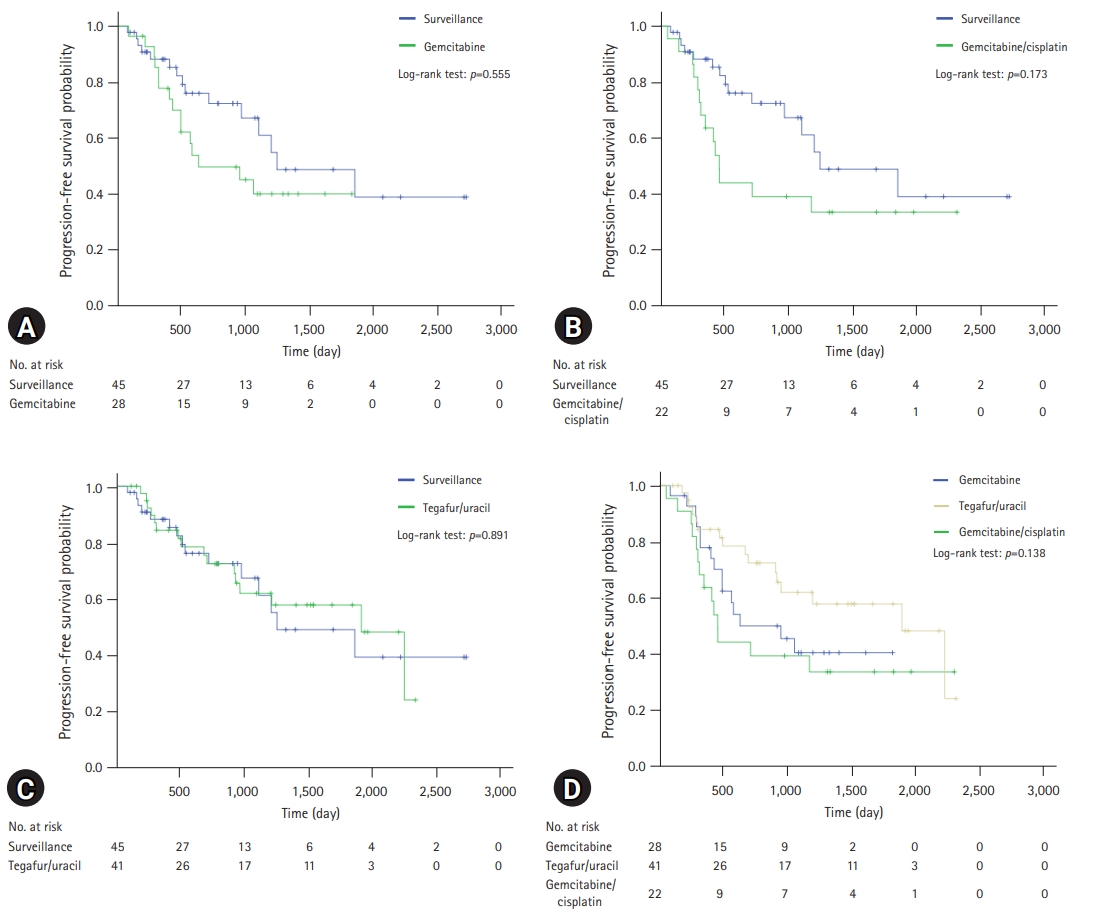
- A comparison of the OS and PFS for all patients is shown in Fig. 2. The comparisons of OS and PFS in the subgroups including lymphovascular invasion, perineural invasion, and AJCC stage are shown in Figs. 3, 4, respectively. AT did not demonstrate a survival benefit in any of the analyses. The comparison of OS according to AT regimens is shown in Fig. 5. The 1-year, 3-year, and 5-year OSRs were 100%, 79.3%, and 69.3% in the gemcitabine group; 90.7%, 67.0%, and 55.8% in the gemcitabine/cisplatin group; and 100%, 86.3%, and 81.5% in the tegafur/uracil group, respectively. The tegafur/uracil group showed the highest OSR among the regimens, although the difference was not statistically significant (p=0.358). However, tegafur/uracil did not demonstrate a survival benefit compared with the surveillance group (p=0.268). A comparison of PFS according to the AT regimens is shown in Fig. 6. Similar to the OSR analysis, the tegafur/uracil group showed the highest PFSR among the regimens, although it was not statistically significant (p=0.138). However, no survival benefit was observed with tegafur/uracil compared with the surveillance group (p=0.891).
- 3. Prognostic factors associated with overall survival and progression-free survival
- In the univariate analysis, CEA elevation (HR, 3.38; 95% CI, 1.30–8.74; p=0.012), lymphovascular invasion (HR, 2.11; 95% CI, 1.10–4.03; p=0.023), and AJCC stage III (HR, 9.81; 95% CI, 2.67–36.10; p<0.001) were useful prognostic factors for OS (Table 3). However, AT was not a statistically significant factor (HR, 0.92; 95% CI, 0.45–1.86; p=0.806). In the multivariate analysis, only AJCC stage III (HR, 10.81; 95% CI, 2.92–40.00; p<0.001) was a statistically significant prognostic factor associated with OS (Table 3). CA 19-9 elevation (HR, 1.80; 95% CI, 0.93–3.52; p=0.083) was useful but not statistically significant. Prognostic factors associated with PFS were analyzed and are shown in Table 4. In the multivariate analysis, ASA classification II (HR, 0.50; 95% CI, 0.31–0.81; p=0.005), AJCC stage II (HR, 3.14; 95% CI, 1.25–7.89; p=0.015), and AJCC stage III (HR, 8.08; 95% CI, 2.80–23.32; p<0.001) were significant prognostic factors for PFS (Table 4). Poorly differentiated cancer was statistically significant in the univariate analysis (HR, 1.92; 95% CI, 1.17–3.16; p=0.010), but not in the multivariate analysis (HR, 1.64; 95% CI, 0.98–2.75; p=0.060). As in the OS analysis, AT was not a statistically significant factor associated with PFS (HR, 1.57; 95% CI, 0.89–2.78; p=0.117).
- 4. Therapeutic adherence to adjuvant therapy according to regimen
- Therapeutic adherence in the AT group according to the regimen is summarized in Table 5. Of the 109 patients who received AT, 72 (66.1%) completed the therapeutic schedule, and 37 (33.9%) did not finish the cycle. The reasons for cessation included recurrence (37.8%), patient refusal (24.3%), neutropenia (8.1%), and loss to follow-up (5.4%). Of the 109 patients who received AT, 36 (33.0%) received second-line therapy.
Results
- To our knowledge, there have been only five large prospective studies on adjuvant chemotherapy for CCA [6-10]. Only two of these studies showed positive results in patients who underwent surgery with curative intent, and these results were only marginally positive. In the ESPAC-3 trial, adjuvant chemotherapy was associated with increased OS in multivariate analysis (HR, 0.75; 95% CI, 0.57–0.98; p=0.03) [7], and in the BILCAP study, the median OS was 53 months in the capecitabine group and 17.5 months in the observation group (HR, 0.75; 95% CI, 0.58–0.97; p=0.028) in per-protocol analysis [8]. In contrast, retrospective studies of AT for CCA showed more positive results [11-18]. However, the subgroups that showed survival benefits differed among studies and included AJCC stage II and III biliary tract cancer [11], R1 resection or lymph node involvement [12], perineural invasion [13], and intrahepatic and AJCC stage III cancer [16]. There are two explanations for the inconsistent results among these studies. First, the CCA subtypes varied in each study. CCA is divided into three subtypes according to location, and each subtype has different characteristics, including risk factors and genetic aberrations [1]. Second, selection bias exists in retrospective studies. In the real world, patients considered to be at high risk of recurrence are likely to receive AT [13].
- In this single-center analysis, AT did not demonstrate survival benefits. There are several possible explanations for this finding. First, all the patients included in this study underwent R0 resection. Previous studies on the effect of AT on R0-resected CCA are summarized in Table 6. Of these studies, four investigated R0 resection only [11,16-18], and the other two addressed it by subgroup analysis [9,20]. This shows that a survival benefit of AT in patients with R0-resected CCA has not been clearly demonstrated. Second, the CCA subtypes included in each study were different. For example, in previous studies in which AT showed positive results, the proportion of gallbladder cancer ranged from 13.6% to 48.3% [11-13,15]. However, patients with gallbladder cancer were not included in the present study. Third, differences in the AT regimen could be an important factor. Among several prospective studies, the only drug that showed an increase in OS was capecitabine [8]. However, none of the patients in the present study received capecitabine. Instead, patients who received tegafur/uracil, which is similar to capecitabine, showed the highest OSR and PFSR among the regimens, although the difference was not statistically significant. Furthermore, patients in the AT group had a more advanced AJCC stage, which was a significant prognostic factor for OS and PFS in this study.
- Another possible explanation for the failure to demonstrate survival benefits is the low AT completion rate. In this study, one-third of the patients who received AT did not complete their planned schedule. This result is similar to that reported in recent studies (26.0%–53.7%) [8,10,15]. The goal of adjuvant chemotherapy is the eradication of micrometastasis, and it is important to achieve complete remission to increase survival [22]. From this perspective, completion of the therapeutic schedule is important. In this study, the second most common cause of cessation was patient refusal (21.7%). Toxicity, including cytopenia and skin eruptions, was also an important cause. These factors can be corrected with meticulous intervention. One example of this is individualized chemotherapy. The standard method for determining the dose of chemotherapy is based on the body surface area calculated using the patient’s weight and height [23]. However, several studies have reported improved clinical outcomes and reduced toxicity with individualized chemotherapy based on pharmacokinetic monitoring of the colorectal cancer [24,25].
- In this study, PFSR tended to be lower in the AT group, although the difference was not statistically significant. This could be explained by two factors. First, the time interval of follow-up CT was shorter in the AT group, which might have allowed for the early detection of disease progression. Second, as noted previously, patients considered to be at high risk of recurrence are likely to receive AT [13]. To overcome this bias, a Cox proportional hazards model was used. In the multivariate analysis, AJCC stage III was a significant factor associated with OS. ASA classification II, AJCC stage II, and AJCC stage III were independent prognostic factors associated with PFS. Advanced AJCC stage was also identified as significant prognostic factor in previous studies [17,19]. In contrast, perineural and lymphovascular invasion were not statistically significant factors in this study. However, it should be taken into consideration that lymphovascular invasion and perineural invasion were unknown in 12.8% and 21.2% of the patients, respectively.
- Interestingly, ASA classification was a favorable prognostic factor for PFS in this study. Chauhan et al. [26] reported that a high preoperative Charlson comorbidity index was an independent risk factor associated with significantly worse PFS (HR, 7.36; 95% CI, 2.68–12.12; p<0.001) in patients with R0-resected perihilar CCA. However, the patients included in the present study were either ASA classification I (normal healthy patients) or ASA classification II (patients with mild systemic disease, such as current smokers or those with obesity, well-controlled diabetes, hypertension, or mild lung disease) [27]. Therefore, additional research on the relationship between mild systemic diseases and PFS is required.
- This study has several limitations. First, this was a retrospective study; thus, quality of life and adverse events were not investigated. The characteristics of both the AT and surveillance groups, including sex, age, and disease stage, were different owing to selection bias. Furthermore, the Eastern Cooperative Oncology Group performance status was not evaluated in most patients; therefore, ASA classification was used in this study instead. Second, it is difficult to generalize the results of this study because it was a single-center study. Third, capecitabine was not administered to the patients in this study. This is because capecitabine was approved by Korean Health Insurance in 2019. Fourth, patients with gallbladder cancer were excluded from the study. Finally, treatment and follow-up were performed at the discretion of the clinician. However, this study is meaningful because we analyzed adherence and the effect of AT in a real-world scenario with a large number of patients.
- In conclusion, AT did not improve OS or PFS in patients with CCA who underwent R0 resection. AJCC stage III was a significant prognostic factor for OS. The prognostic factors associated with PFS were ASA classification II, AJCC stage II, and AJCC stage III. After adjusting for confounding variables using the Cox proportional hazards model, AT did not show any survival benefit. However, we found that AT was not completed in one-third of patients. Therefore, additional efforts are required to increase adherence to AT.
Discussion
-
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
-
Funding
None.
-
Author contributions
Conceptualization: all authors; Data curation: JL; Formal analysis: all authors; Methodology, Supervision: HGK, JH; Investigation: HTJ, JL, HHJ; Writing-original draft: HTJ; Writing-review & editing: HTJ, HHJ, HGK, JH.
Notes

| Characteristic | Total | Surveillance | Adjuvant therapy | p-value |
|---|---|---|---|---|
| No. of patients | 154 | 45 (29.2) | 109 (70.8) | |
| Age (yr) | 70 (61–74) | 74 (68–77) | 67 (60–73) | <0.001 |
| Male sex | 91 (59.1) | 21 (46.7) | 70 (64.2) | 0.044 |
| ASA classification | 0.740 | |||
| I | 45 (29.2) | 14 (31.1) | 31 (28.4) | |
| II | 109 (70.8) | 31 (68.9) | 78 (71.6) | |
| Tumor marker | ||||
| CA 19-9 (U/mL) | 40 (15–139) | 50 (17–109) | 35 (14–172) | 0.725 |
| CEA (ng/mL) | 2.8 (2.0–4.8) | 2.9 (2.0–5.1) | 2.7 (2.0–4.5) | 0.526 |
| Location | 0.187 | |||
| Intrahepatic | 29 (18.8) | 9 (20.0) | 20 (18.3) | |
| Perihilar | 43 (27.9) | 8 (17.8) | 35 (32.1) | |
| Distal | 82 (53.2) | 28 (62.2) | 54 (49.5) | |
| Pathologic classification | 0.969 | |||
| Adenocarcinoma | 149 (96.8) | 43 (95.6) | 106 (97.2) | |
| Othersa) | 5 (3.2) | 2 (4.4) | 3 (2.8) | |
| Histologic differentiation | 0.526 | |||
| Well | 22 (14.3) | 6 (13.3) | 16 (14.7) | |
| Moderately | 87 (56.5) | 29 (64.4) | 58 (53.2) | |
| Poorly | 39 (25.3) | 8 (17.8) | 31 (28.4) | |
| Unknown | 6 (3.9) | 2 (4.4) | 4 (3.7) | |
| AJCC stage | 0.005 | |||
| I | 25 (16.2) | 13 (28.9) | 12 (11.0) | |
| II | 113 (73.4) | 31 (68.9) | 82 (75.2) | |
| III | 16 (10.4) | 1 (2.2) | 15 (13.8) | |
| Lymphovascular invasion | 0.904 | |||
| Negative | 81 (52.6) | 24 (53.3) | 57 (52.3) | |
| Positive | 53 (34.4) | 16 (35.6) | 37 (33.9) | |
| Unknown | 20 (13.0) | 5 (11.1) | 15 (13.8) | |
| Perineural invasion | 0.254 | |||
| Negative | 30 (19.5) | 11 (24.4) | 19 (17.4) | |
| Positive | 91 (59.1) | 22 (48.9) | 69 (63.3) | |
| Unknown | 33 (21.4) | 12 (26.7) | 21 (19.3) | |
| Regimen | N/A | |||
| Tegafur/uracil | 41 (26.6) | 0 (0) | 41 (37.6) | |
| Gemcitabine/cisplatin | 22 (14.3) | 0 (0) | 22 (20.2) | |
| Gemcitabine only | 28 (18.2) | 0 (0) | 28 (25.7) | |
| Othersb) | 18 (11.7) | 0 (0) | 18 (16.5) |
Values are presented as number (%) or median (interquartile range).
ASA, American Society of Anesthesiologists; CA 19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; AJCC, American Joint Committee on Cancer; N/A, not applicable.
a) Others include three adenosquamous carcinomas, one mixed adenoneuroendocrine carcinoma, and one undifferentiated carcinoma.
b) Others include 12 fluorouracil/radiotherapy and six fluorouracil/cisplatin.
| Variable | Total (n=109) | Gemcitabine based (n=50) | Othersa) (n=59) |
|---|---|---|---|
| Cessation | 37 (33.9) | 12 (24.0) | 25 (42.4) |
| Cause for cessation | |||
| Recurrence | 14 (37.8) | 2 (16.7) | 12 (48.0) |
| Refusal | 9 (24.3) | 5 (41.7) | 4 (16.0) |
| Neutropenia | 3 (8.1) | 3 (25.0) | 0 (0) |
| Follow-up loss | 2 (5.4) | 0 (0) | 2 (8.0) |
| Skin eruption | 2 (5.4) | 1 (8.3) | 1 (4.0) |
| Othersb) | 7 (18.9) | 1 (8.3) | 6 (24.0) |
| Second-line therapy | 36 (33.0) | 24 (48.0) | 12 (20.3) |
| Reference | Design | Location | Regimen | No. of patients |
Overall survival |
p-value | |
|---|---|---|---|---|---|---|---|
| Median (mo) | 5-Yr OSR (%) | ||||||
| Ebata et al. [9] | Prospective | BD | AT | 106 | NA | 60a) | 0.965 |
| Surveillance | 94 | NA | 60a) | ||||
| Kim et al. [11] | Retrospective | GB, BD | AT | 89 | NA | 52.4 | 0.002 |
| Surveillance | 64 | NA | 35.4 | ||||
| Yamanaka et al. [16] | Retrospective | GB, BD, AoV | AT | 40 | NA | 68.0b) | N/A |
| Surveillance | 158 | NA | 68.7b) | ||||
| Kim et al. [17] | Retrospective | BDc) | AT, CRT, RT | 56 | 72.9 | NA | 0.172 |
| Surveillance | 102 | 51.1 | NA | ||||
| Kim et al. [18] | Retrospective | BDd) | AT, CRT, RT | 73 | 24.7 | NA | 0.019 |
| Surveillance | 64 | 47.3 | NA | ||||
| Yin et al. [20] | Retrospective | GB, BD, AoV | AT | 40 | 33.7 | 37.9b) | 0.114 |
| Surveillance | 40 | 21.1 | 21.1b) | ||||
| This study | Retrospective | BD | AT, CRT, RT | 111 | NA | 63.4 | 0.878 |
| Surveillance | 45 | NA | 63.4 | ||||
OSR, overall survival rate; BD, bile duct; AT, adjuvant therapy; N/A, not applicable. GB, gallbladder; AoV, ampulla of Vater; CRT, chemoradiotherapy; RT, radiotherapy.
a) This is an approximate number obtained from the graph.
b) This is a 3-year OSR.
c) This includes distal cholangiocarcinoma.
d) This includes intrahepatic and perihilar cholangiocarcinoma.
- 1. Brindley PJ, Bachini M, Ilyas SI, Khan SA, Loukas A, Sirica AE, et al. Cholangiocarcinoma. Nat Rev Dis Primers 2021;7:65.ArticlePubMedPMCPDF
- 2. Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: epidemiology and risk factors. Liver Int 2019;39(Suppl 1):19–31.ArticlePubMedPDF
- 3. Belkouz A, Wilmink JW, Haj Mohammad N, Hagendoorn J, de Vos-Geelen J, Dejong CH, et al. Advances in adjuvant therapy of biliary tract cancer: an overview of current clinical evidence based on phase II and III trials. Crit Rev Oncol Hematol 2020;151:102975.ArticlePubMed
- 4. Allen MJ, Knox JJ. A review of current adjuvant and neoadjuvant systemic treatments for cholangiocarcinoma and gallbladder carcinoma. Hepatoma Res 2021;7:73.Article
- 5. Kim BW, Oh CM, Choi HY, Park JW, Cho H, Ki M. Incidence and overall survival of biliary tract cancers in South Korea from 2006 to 2015: using the National Health Information Database. Gut Liver 2019;13:104–13.ArticlePubMedPMC
- 6. Takada T, Amano H, Yasuda H, Nimura Y, Matsushiro T, Kato H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma?: a phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer 2002;95:1685–95.ArticlePubMed
- 7. Neoptolemos JP, Moore MJ, Cox TF, Valle JW, Palmer DH, McDonald AC, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA 2012;308:147–56.ArticlePubMed
- 8. Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol 2019;20:663–73.ArticlePubMed
- 9. Ebata T, Hirano S, Konishi M, Uesaka K, Tsuchiya Y, Ohtsuka M, et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br J Surg 2018;105:192–202.PubMed
- 10. Edeline J, Benabdelghani M, Bertaut A, Watelet J, Hammel P, Joly JP, et al. Gemcitabine and oxaliplatin chemotherapy or surveillance in resected biliary tract cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): a randomized phase III study. J Clin Oncol 2019;37:658–67.ArticlePubMed
- 11. Kim YS, Jeong CY, Song HN, Kim TH, Kim HJ, Lee YJ, et al. The efficacy of fluoropyrimidine-based adjuvant chemotherapy on biliary tract cancer after R0 resection. Chin J Cancer 2017;36:9.ArticlePubMedPMCPDF
- 12. McNamara MG, Walter T, Horgan AM, Amir E, Cleary S, McKeever EL, et al. Outcome of adjuvant therapy in biliary tract cancers. Am J Clin Oncol 2015;38:382–7.ArticlePubMed
- 13. Miyata Y, Kogure R, Nakazawa A, Nagata R, Mitsui T, Ninomiya R, et al. The efficacy of S-1 as adjuvant chemotherapy for resected biliary tract carcinoma: a propensity score-matching analysis. J Clin Med 2021;10:925.ArticlePubMedPMC
- 14. Wang L, Deng M, Ke Q, Lou J, Zheng S, Bi X, et al. Postoperative adjuvant therapy following radical resection for intrahepatic cholangiocarcinoma: a multicenter retrospective study. Cancer Med 2020;9:2674–85.ArticlePubMedPMCPDF
- 15. Murakami Y, Uemura K, Sudo T, Hayashidani Y, Hashimoto Y, Nakamura H, et al. Adjuvant gemcitabine plus S-1 chemotherapy improves survival after aggressive surgical resection for advanced biliary carcinoma. Ann Surg 2009;250:950–6.ArticlePubMed
- 16. Yamanaka K, Hatano E, Kanai M, Tanaka S, Yamamoto K, Narita M, et al. A single-center analysis of the survival benefits of adjuvant gemcitabine chemotherapy for biliary tract cancer. Int J Clin Oncol 2014;19:485–9.ArticlePubMedPDF
- 17. Kim YS, Hwang IG, Park SE, Go SI, Kang JH, Park I, et al. Role of adjuvant therapy after R0 resection for patients with distal cholangiocarcinoma. Cancer Chemother Pharmacol 2016;77:979–85.ArticlePubMedPDF
- 18. Kim YS, Oh SY, Go SI, Kang JH, Park I, Song HN, et al. The role of adjuvant therapy after R0 resection for patients with intrahepatic and perihilar cholangiocarcinomas. Cancer Chemother Pharmacol 2017;79:99–106.ArticlePubMedPDF
- 19. Bergeat D, Turrini O, Courtin-Tanguy L, Truant S, Darnis B, Delpero JR, et al. Impact of adjuvant chemotherapy after pancreaticoduodenectomy for distal cholangiocarcinoma: a propensity score analysis from a French multicentric cohort. Langenbecks Arch Surg 2018;403:701–9.ArticlePubMedPDF
- 20. Yin L, Xu Q, Li J, Wei Q, Ying J. The efficiency and regimen choice of adjuvant chemotherapy in biliary tract cancer: a STROBE-compliant retrospective cohort study. Medicine (Baltimore) 2018;97:e13570.ArticlePubMedPMC
- 21. National Comprehensive Cancer Network (NCCN). NCCN Practice Guidelines in Oncology. Hepatobiliary cancers, version 5 [Internet]. Plymouth Meeting (PA): NCCN; 2021 [cited 2021 Sep 21]. https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf.
- 22. Ringborg U. Adjuvant chemotherapy: a discussion of some basic principles. Acta Oncol 1991;30:251–3.ArticlePubMed
- 23. Mosteller RD. Simplified calculation of body-surface area. N Engl J Med 1987;317:1098.ArticlePubMed
- 24. Saam J, Critchfield GC, Hamilton SA, Roa BB, Wenstrup RJ, Kaldate RR. Body surface area-based dosing of 5-fluoruracil results in extensive interindividual variability in 5-fluorouracil exposure in colorectal cancer patients on FOLFOX regimens. Clin Colorectal Cancer 2011;10:203–6.ArticlePubMed
- 25. Capitain O, Asevoaia A, Boisdron-Celle M, Poirier AL, Morel A, Gamelin E. Individual fluorouracil dose adjustment in FOLFOX based on pharmacokinetic follow-up compared with conventional body-area-surface dosing: a phase II, proof-of-concept study. Clin Colorectal Cancer 2012;11:263–7.ArticlePubMed
- 26. Chauhan A, House MG, Pitt HA, Nakeeb A, Howard TJ, Zyromski NJ, et al. Post-operative morbidity results in decreased long-term survival after resection for hilar cholangiocarcinoma. HPB (Oxford) 2011;13:139–47.ArticlePubMedPMC
- 27. Hurwitz EE, Simon M, Vinta SR, Zehm CF, Shabot SM, Minhajuddin A, et al. Adding examples to the ASA-Physical Status Classification improves correct assignment to patients. Anesthesiology 2017;126:614–22.ArticlePubMedPDF
References
Figure & Data
References
Citations

- Robotic Complete ALPPS (rALPPS)—First German Experiences
Jörg Arend, Mareike Franz, Alexander Rose, Christine March, Mirhasan Rahimli, Aristotelis Perrakis, Eric Lorenz, Roland Croner
Cancers.2024; 16(5): 1070. CrossRef
- Figure
- Related articles
-
- Aortic valve replacement through right anterior mini-thoracotomy in patients with chronic severe aortic regurgitation: a retrospective single-center study
- Effect of prehydration solution on hearing threshold after chemotherapy in patients with head and neck cancers: a retrospective study
- Clinical impact of spine magnetic resonance imaging as a valuable prognostic tool for patients with multiple myeloma: a retrospective study
- Clinical implication of adjuvant chemotherapy according to mismatch repair status in patients with intermediate-risk stage II colon cancer: a retrospective study
- The clinical outcomes of second-line chemotherapy in patients with advanced pancreatic cancer: a retrospective study

 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine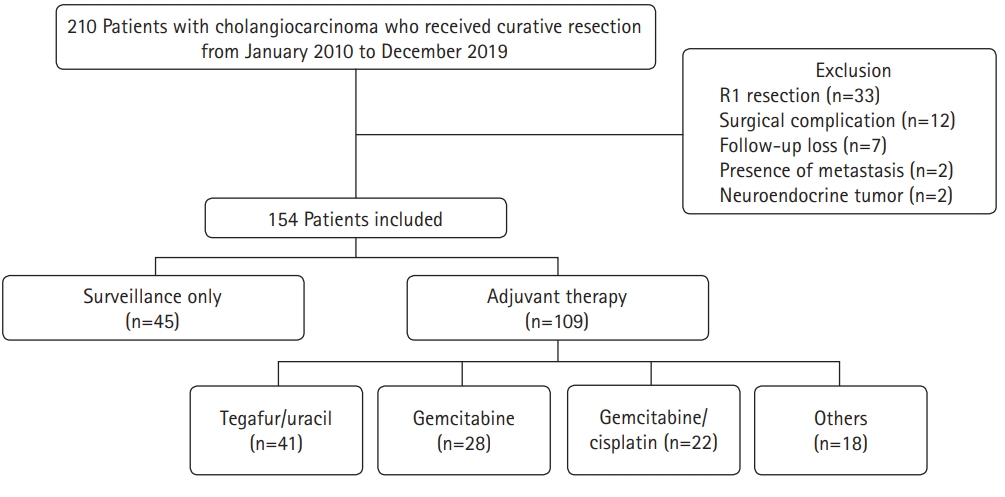
 PubReader
PubReader ePub Link
ePub Link Cite
Cite