PubMed Central, CAS, DOAJ, KCI

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Ahead-of print > Article
-
Review article
Clinical significance of exosomal noncoding RNAs in hepatocellular carcinoma: a narrative review -
Jae Sung Yoo1
 , Min Kyu Kang2
, Min Kyu Kang2
-
DOI: https://doi.org/10.12701/jyms.2023.01186
Published online: February 8, 2024
1Department of Gastroenterology and Hepatology, The Catholic University of Korea, Seoul St Mary’s Hospital, Seoul, Korea
2Department of Internal Medicine, Yeungnam University College of Medicine, Daegu, Korea
- Corresponding author: Min Kyu Kang, MD Department of Internal Medicine, Yeungnam University College of Medicine, 170 Hyeonchung-ro, Nam-gu, Daegu 42415, Korea Tel: +82-53-620-3316 • Fax: +82-53-654-8386 • E-mail: kmggood111@naver.com
© 2024 Yeungnam University College of Medicine, Yeungnam University Institute of Medical Science
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,117 Views
- 31 Download
Abstract
- Hepatocellular carcinoma (HCC) is one of the most lethal malignancies worldwide, with poor prognosis owing to its high frequency of recurrence and metastasis. Moreover, most patients are diagnosed at an advanced stage owing to a lack of early detection markers. Exosomes, which are characterized by their cargos of stable intracellular messengers, such as DNA, RNA, proteins, and lipids, play a crucial role in regulating cell differentiation and HCC development. Recently, exosomal noncoding RNAs (ncRNAs), including microRNAs, long ncRNAs, and circular RNAs, have become increasingly important diagnostic, prognostic, and predictive markers of HCC. Herein, we discuss the clinical implications of exosomal ncRNAs, specifically those within the HCC regulatory network.
- Hepatocellular carcinoma (HCC) is a significant health issue for patients with chronic liver disease, has a high socioeconomic burden, and is the third leading cause of cancer-related mortality worldwide [1,2]. Patients diagnosed with HCC in the early stage, accounting for 30% to 40% of all HCC cases, have a favorable prognosis because of the feasibility of curative treatments, which include liver transplantation, surgical resection, and local ablation therapy [3]. However, most patients with HCC diagnosed at intermediate or advanced stages have a poor prognosis due to high rates of recurrence and metastasis, as well as the limitations of curative treatments [3-6].
- In general, current diagnostic methods for detecting HCC rely on clinical radiological modalities, such as computed tomography and magnetic resonance imaging, without the need for histological confirmation because of the potential of liver biopsies to cause serious complications, including bleeding, cancer seeding, rupture, and even death [7,8]. Nonetheless, the sensitivity of radiological modalities for early detection of HCC varies based on the type of modality and diagnostic criteria applied [9,10]. As a serologic diagnostic marker, α‐fetoprotein (AFP) is considered a novel biomarker for early identification of patients with HCC. However, the sensitivity of AFP for detecting HCC is low at 20% to 65% [11].
- Recent studies have indicated that exosomal noncoding RNAs (ncRNAs) are potential diagnostic and prognostic markers in patients with HCC. Previously, ncRNAs were considered nonfunctional genes. However, with the development of RNA identification techniques such as next-generation sequencing, various ncRNAs have been shown to regulate target messenger RNAs (mRNAs) and modulate gene expression [12,13]. Recently, dysregulation of ncRNAs has been linked to tumor progression and metastasis in patients with HCC, and ncRNAs are now considered to be promising diagnostic and therapeutic targets for HCC [14].
- Based on their nucleotide length and structure, ncRNAs are classified into microRNAs (miRNAs), long ncRNAs (lncRNAs), and circular RNAs (circRNAs). MiRNAs are single-stranded ncRNAs of 19–25 nucleotides that are closely associated with various biological processes and regulate protein expression by mRNA degradation and translational inhibition by binding to the 3′-untranslated region of target pre-mRNAs [15,16]. Given that miRNAs can function as either tumor suppressors or oncogenes, their aberrant expression has been linked to carcinogenesis, including tumor angiogenesis, cell proliferation, tumor invasion, and metastasis [17-20]. LncRNAs, which are ncRNAs exceeding 200 nucleotides in length and typically do not code for proteins, are known for their heterogeneity [21]. Previous studies have indicated that lncRNAs regulate gene expression by acting as miRNA sponges or competing endogenous RNAs (ceRNAs) [22,23]. Their dysregulation has been found to affect carcinogenic processes, including cell differentiation and proliferation, by altering gene expression [24,25]. CircRNAs are endogenous circular ncRNAs with covalently closed linked ends (in contrast to linear RNAs such as miRNAs and lncRNAs) and are produced by the backsplicing of exons and/or introns on precursor mRNAs [26]. Unlike linear RNAs, circRNAs are resistant to RNase because they lack a 5′ cap and 3′ polyadenylated tail [27]. Similar to lncRNAs, the precise roles of circRNAs remain unclear. However, circRNAs has been reported to function as miRNA sponges or ceRNAs at both transcriptional and posttranscriptional levels [28].
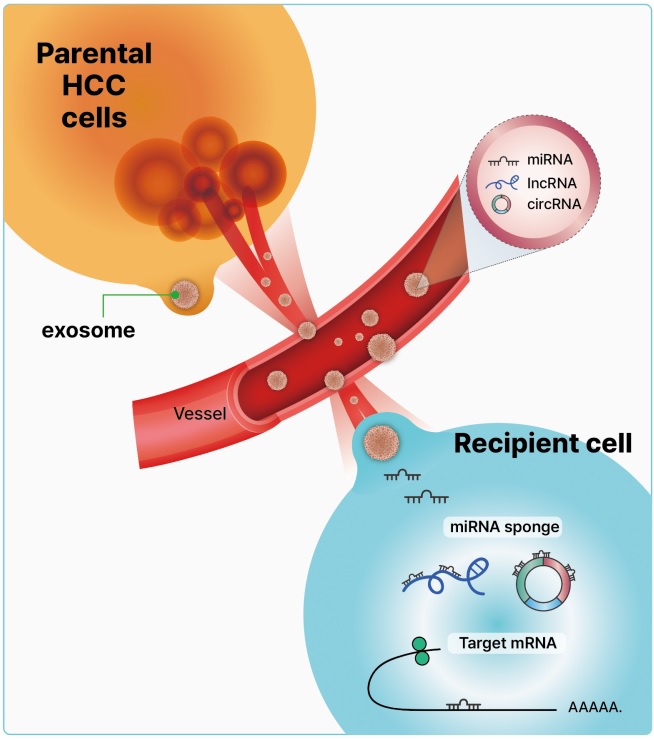
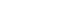
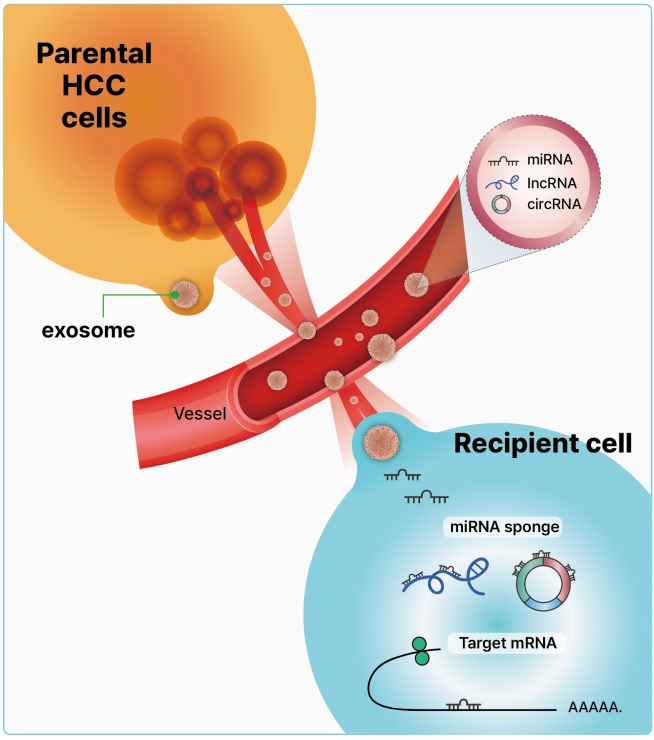
- Exosomes are nanoscale cup-shaped or double concave disc-shaped vesicles (30–100 nm) secreted by most cell types; they can be detected in body fluids, such as serum, urine, ascitic fluid, and breast milk [29-33]. Exosomes play a crucial role in cell-to-cell communication between donor and recipient cells by fusing with cell receptors and transferring genetic information [34]. Exosomes contain cell-specific mRNAs, ncRNAs (including miRNAs, lncRNAs, and circRNAs), and proteins, which are stabilized in the circulation because of their exosomal protective and antidegradative functions against RNase [35-38] (Fig. 1). Recent study have demonstrated that exosomal miRNAs may serve as potential diagnostic and prognostic markers in patients with malignancies [39].
- Given the clinical importance of early detection for improving prognosis and facilitating curative treatment of HCC, the exploration of emerging diagnostic and prognostic biomarkers is warranted [40]. Although exosomal ncRNAs are promising novel biomarkers of HCC, their mechanisms of action in patients with HCC remain unclear. In this review, we describe the clinical applications of HCC-derived exosomal miRNAs, lncRNAs, and circRNAs over the past decade and classify them into diagnostic, prognostic, and therapeutic categories.
Introduction
- Early detection of HCC can improve curative treatment options and long-term survival rates. Traditional diagnostic methods for HCC have limited sensitivity, making early detection challenging. Currently, a significant amount of research is focused on liquid biopsy techniques as new diagnostic methods. Recent studies have shown promising results regarding the use of exosomal ncRNAs as effective diagnostic markers in patients with HCC. Through the advancement of purification and isolation techniques for miRNAs using RNA extraction kits, exosomal miRNAs have been specifically identified as diagnostic marker, when compared to exosomal lncRNAs and circRNAs. Table 1 [41-68] summarizes the diagnostic markers of exosomal ncRNAs in patients with HCC.
- 1. Exosomal microRNAs as diagnostic biomarkers
- Previous research has identified elevated levels of plasma miRNA-21 even in the early stages of HCC. Further analysis revealed that the level of plasma miRNA-21 was significantly higher in patients with HCC than in those with chronic hepatitis or in the control group. This study is the first to demonstrate the feasibility of using plasma miRNA-21 as an early diagnostic marker for HCC [69]. Wang et al. [41] demonstrated that exosomal miRNA-21 is a potential diagnostic marker for early HCC. Their study found that serum exosomal miRNA-21 expression was higher in patients with HCC than in healthy individuals and in patients with chronic hepatitis B (CHB). Additionally, exosomal miRNA-21 expression levels were found to be higher than those in exosome-depleted serum and whole serum [41]. However, this study did not identify the target genes associated with HCC.
- Owing to the low sensitivity of AFP in the diagnosis of HCC, it has been proposed that the combination of an emerging exosomal miRNA with AFP can enhance the accuracy of HCC diagnosis. Liu et al. [42] identified promising diagnostic biomarkers for non–virus-infected HCC in diethylnitrosamine-induced HCC rats. Specifically, four selected miRNAs (miRNA-10b and miRNA-21 as oncogenes and miRNA-122 and miRNA-200a as tumor suppressor genes) were identified. The combination of exosomal miRNAs and AFP exhibited a more significant diagnostic potential in predicting HCC as compared to the use of AFP alone [42]. Wang et al. [43] found that the serum exosomal levels of miRNA-122, miRNA-148a, and miRNA-1246 were significantly higher in individuals with HCC than in those with liver cirrhosis (LC) or in normal controls (NC). A combination of serum exosomal miRNA-122, miRNA-148a, and AFP levels can be used to discriminate between early HCC and LC. While plasma miRNA-21 is not exosome-derived, a previous study demonstrated that the combination of plasma miRNA-21 and AFP has stronger discriminant power for detecting early HCC than AFP alone [69].
- In a domestic study, Sohn et al. [44] found that the use of variable serum exosomal miRNAs resulted in improved diagnostic accuracy for distinguishing HCC from CHB or LC when compared to serum circulating miRNAs. In that study, researchers observed higher serum levels of exosomal miRNA-18a, miRNA-221, miRNA-222, and miRNA-224 and lower levels of exosomal miRNA-101, miRNA-106b, miRNA-122, and miRNA-195 in patients with HCC than in those with CHB or LC. Compared to previous studies, exosomal miRNA expression was similar to that of tissue miRNAs. In HCC tissues, miRNA-18a, miRNA-221, miRNA-222, and miRNA-224 were overexpressed, whereas miRNA-101, miRNA-195, and miRNA-122a were downregulated [70-72]. However, the target genes of these miRNAs in HCC were not identified in the study. Interestingly, serum circulating miRNAs were not significantly different between the HCC and CHB groups, unlike serum exosomal miRNAs. This study suggests that serum exosomal miRNAs are more effective markers than circulating serum miRNAs for differentiating HCC from CHB or LC [44].
- Cho et al. [45] found that serum exosomal miRNA-10b-5p is a potential biomarker for early-stage HCC, with an area under the curve (AUC) of 0.934. Han et al. [46] found that miRNA-148a expression in the plasma was significantly decreased in individuals with HCC. When comparing patients with HCC to those with LC, the AUC for plasma miRNA-148a was 0.919 (sensitivity, 89.5%; specificity, 89.5%). In patients with HCC and low or negative AFP, the AUC for plasma miRNA-148a was 0.949 (sensitivity, 90.6%; specificity, 92.6%). MiRNA-148a could be a feasible noninvasive biomarker that may work in conjunction with AFP to diagnose HCC. Fu et al. [47] demonstrated the crucial role of phosphatase and tensin homolog (PTEN) and miRNA-155-5p in aggressive HCC both in vitro and in vivo. In a diethylnitrosamine/N-nitrosomorpholine-induced HCC rat model, miRNA-155-5p overexpression occurred simultaneously with PTEN mRNA suppression. These findings have also been observed in human HCC tissues and cell lines. Therefore, miRNA-155-5p may serve as a novel diagnostic marker and therapeutic target.
- 2. Exosomal long noncoding RNAs and circular RNAs as diagnostic biomarkers
- Recently, research has been conducted to identify not only the basic functions of miRNAs, but also lncRNAs and circRNAs, which can act as miRNA sponges and regulate target genes in patients with HCC. Specifically, circRNAs are resistant to exonuclease degradation because of their covalently closed linked ends, leading to their accumulation in cells and slow degradation [73]. Furthermore, exosomes can protect lncRNAs from degradation by RNase, allowing them to remain stable within exosomes [29]. However, compared with investigations of exosomal miRNAs, those of exosomal lncRNAs and circRNAs are rare in patients with HCC. Table 1 lists the diagnostic biomarkers of the exosomal lncRNAs and circRNAs identified in HCC.
- Zhang et al. [48] demonstrated that patients with hepatitis C virus-related HCC have elevated expression levels of lncRNA-HEIH in serum and exosomes, indicating its potential as a biomarker for the diagnosis of HCC. However, the ratio of lncRNA-HEIH in serum versus exosomes is lower in patients with HCC than in those with chronic hepatitis C (CHC). The authors hypothesized that the exosomal lncRNA has limited potential to regulate gene expression until its release from exosomes into the serum. Xie et al. [49] demonstrated a difference in the expression of lncRNA-highly upregulated in liver cancer (HULC) between the tissues and plasma of patients with HCC and healthy controls. The AUC of lncRNA-HULC in predicting HCC was 0.86. Moreover, these data suggest that lncRNA-HULC is associated with malignancy and tumor stage. These results show that lncRNA-HULC expression in the plasma may be used as a noninvasive, innovative biomarker that shows promise for the diagnosis and prognosis of HCC. Wang et al. [50] revealed that miRNA-186 is sequestered by lncRNA-HULC, increasing high-mobility group AT-hook 2 expression, thereby promoting the progression of liver cancer. Ji et al. [51] showed that linc00152 may contribute to the oncogenesis of HCC and may eventually serve as a potential indicator of clinical outcomes by stimulating the mammalian target of rapamycin signaling pathway. Additionally, Kim et al. [52] demonstrated that serum extracellular vesicle-derived linc00853, which was shown to have a sensitivity of 93.75% and specificity of 89.77%, may be a breakthrough promising early HCC surveillance biomarker. Sun et al. [53] reported that lncRNA-00161 levels were higher in patients with HCC than in controls. The expression of lncRNA-00161 was detected in serum exosomes, exosome-free serum, and urine samples, whereas only serum exosomal lncRNA-00161 was overexpressed in patients with HCC as compared to expression in control patients. These findings indicate that circulating serum exosomal lncRNA-00161 may serve as a biomarker for HCC. However, the target genes and associated miRNAs in patients with HCC were not elucidated in this study.
- Similar to studies on exosomal miRNA, Xu et al. [54] conducted research on the diagnostic value of combining lncRNA and AFP for HCC. They demonstrated that a combination of serum exosomal lncRNA-ENSG00000258332.1, lncRNA-00635, and AFP may be a potential diagnostic marker for HCC. The AUC for the combination of the two lncRNAs and serum AFP was 0.894, while lncRNA-ENSG00000258332.1 and lncRNA-00635 showed AUCs of 0.719 and 0.750, respectively.
- As the regulatory functions of lncRNAs as miRNA sponges or ceRNAs have been elucidated, several studies have recently examined the target miRNAs and genes associated with lncRNAs. Matboli et al. [55] found that serum exosomal lncRNA-RP11-583F2.2 levels were higher in patients with HCC than in those with CHC and in the normal group. The study also found a negative correlation between lncRNA-RP11-583F2.2 and miRNA-1298, which is believed to act as a sponge for miRNA-1298. Furthermore, lncRNA-RP11-583F2.2 demonstrated higher sensitivity and specificity than AFP (sensitivity, 98.3% vs. 90%; specificity, 91.7% vs. 85%). However, the authors did not discuss the putative mechanisms of the target genes. Gao et al. [56] showed that tumor size, TNM stage, and AFP levels were associated with upregulated plasma SNHG1 expression levels. According to receiver operating characteristic analysis, SNHG1 had a great diagnostic performance (AUC, 0.86–0.97) for distinguishing between patients with HCC and healthy control individuals. Li et al. [74] illustrated the mechanism of sorafenib resistance using lncRN-SNHG1. miR-21, whose nuclear translocation is triggered by sorafenib, promotes the nuclear expression of lncRNA-SNHG1, which in turn leads to sorafenib resistance by activating the Akt pathway. These findings indicate that SNHG1 could be a beneficial target for the treatment of patients with HCC that has developed sorafenib resistance.
- Li et al. [57] found significant upregulation of serum exosomal lncRNA-FAL1 in patients with HCC. Regarding its oncogenic role, lncRNA-FAL1 has been reported to promote cell proliferation, invasion, and epithelial-mesenchymal transition (EMT) by increasing the expression of AFP and zinc finger E-box-binding homeobox 1 (ZEB1). Exosomal lncRNA-FAL1 enhances cancer cell proliferation and metastasis by acting as a miR-1236 sponge. In a previous study, miRNA-1236 was found to downregulate HCC proliferation, migration, and invasion as a tumor suppressor, inhibiting the phosphoinositide 3-kinase (PI3K)/Akt pathway through elevated PTEN expression via the repression of AFP [75]. Wang et al. [58] showed that lncRNA-UCA1 promotes the malignant development of human HCC and identified a new regulatory cascade for the UCA1-miRNA-216b-FGFR1-extracellular signal-regulated kinase (ERK) signaling pathway in HCC. These findings are relevant for potential HCC diagnostic markers and/or therapies. Hou et al. [59] revealed that lncRNA-metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) stimulates HCC migration and invasion via two different mechanisms: it sponges miRNA-204 and releases NAD-dependent deacetylase sirtuin-1 (SIRT1) from miRNA-204. Furthermore, through its interaction with miRNA-200a in hypoxic Hep3B cells, MALAT1 is engaged in proliferation, migration, invasion, and apoptosis, revealing a novel mechanism of its involvement in the development of hypoxic HCC [60]. According to Cao et al. [61], lncRNA-UBE2CP3 induces EMT, which in turn enhances HCC metastasis. Additionally, they demonstrated that patients with HCC had elevated blood levels of lncRNA-UBE2CP3. This study determined the significance of lncRNA-UBE2CP3 in predicting HCC development and clinical outcomes. Based on these findings, lncRNA-UBE2CP3 may serve as a potential diagnostic biomarker. Jing et al. [62] showed that lncRNA-SPRY4-IT1 is a key player in the carcinogenesis of HCC and that SPRY4-IT1 might be used as a diagnostic marker for the disease. Nevertheless, these reports did not describe the target genes and related miRNAs in patients with HCC. Huang et al. [63] compared the diagnostic performance of lncRNAs such as HULC, linc00152, UCA1, MALAT1, UBE2CP3, and SPRY4-IT1 using AUC values, and the respective AUC values were 0.796 (95% confidence interval [CI], 0.734–0.858), 0.895 (95% CI, 0.854–0.936), 0.858 (95% CI, 0.810–0.907), 0.768 (95% CI, 0.706–0.830), 0.812 (95% CI, 0.754–0.866), and 0.808 (95% CI, 0.750–0.866), respectively.
- MiRNA-324-5p overexpression serves as a tumor suppressor that decreases migration and invasion in HCC by regulating the expression of matrix metalloproteinase (MMP) 2, MMP9, erythroblast transformation-specific (ETS) 1, and specificity protein 1 [76]. Huang et al. [64] reported that plasma exosomal lncRNA-85 functions as an oncogene and potentially acts as a sponge for miRNA-324-5p, thereby playing a significant role in HCC tumorigenesis. This suggests that lncRNA-85 is a potential biomarker for HCC. Interestingly, the high expression level of exosomal lncRNA-85 is a distinguishing marker for patients with AFP-negative HCC compared to NC and patients with LC, with an AUC value of 0.869 [64].
- There is one report on the use of exosomal circRNAs as diagnostic markers in patients with HCC. Chen et al. [65] illustrated that elevated levels of plasma exosomal circ-0051443 serve as a suitable distinguishing flag for patients with HCC, in contrast to NC, exhibiting an AUC value of 0.8089. Zhang et al. [66] demonstrated that circ_104075 is elevated in HCC and functions as a ceRNA that absorbs miR-582-3p and increases yes-associated protein (YAP) production, thereby stimulating HCC carcinogenesis. Circ_104075 was shown to have a higher sensitivity of 96.0% and specificity of 98.3% for the diagnosis of HCC than other ncRNA biomarkers. Circ _104075 could be a new therapeutic target and diagnostic biomarker for HCC. According to Li et al. [67], circSMARCA5 triggers apoptosis and hinders the growth, invasion, and metastasis of HCC cells. CircSMARCA5 may be a useful biomarker for HCC monitoring and prediction, particularly in patients with AFP levels of <200 ng/mL. Recently, it was reported that a network of noncoding regulatory RNAs, driven by circ‐chromodomain Y like (circ‐CDYL), functioned specifically in the early stages of HCC. The characteristics of liver tumor-initiating cells expressing epithelial cell adhesion molecule (EpCAM) were enhanced by circ-CDYL. Using miRNA-892a and miRNA-328-3p as sponges, circ-CDYL cooperates with mRNAs encoding hepatoma-derived growth factor and hypoxia-inducible factor asparagine hydroxylase. These results reveal a noncoding regulatory RNA system that is circRNA-driven in the early stages of HCC, providing opportunities for early detection and therapy [68].
Diagnostic markers of the exosomal noncoding RNAs of hepatocellular carcinoma
- Several studies have indicated that exosomal ncRNAs are potential prognostic factors in patients with HCC. Our focus is on the clinical implications of exosomal ncRNAs in relation to tumor characteristics such as stage, vessel invasion, metastasis, and target gene expression. Table 2 [41,65,77-93] lists the exosomal ncRNAs known as prognostic markers.
- 1. Exosomal microRNAs as prognostic markers
- In a previous study, high expression of circulating miRNA-21 was linked to PTEN/Akt signaling activation, which promotes EMT and tumor progression, and is associated with poor prognosis in patients with HCC [69]. Wang et al. [41] discovered that high levels of serum exosomal miRNA-21 were positively correlated with LC and advanced tumor stage, indicating that serum exosomal miRNA-21 could be a potential predictive marker for HCC risk. However, this study did not mention the target genes or signaling pathways involved.
- The mitogen-activated protein kinase (MAPK)/ERK signaling pathway is considered an antiapoptotic pathway that plays a vital role in the malignant proliferation of tumor cells by regulating apoptosis-related proteins [94,95]. Recent studies have indicated that MAPK/ERK-related molecules are active in both gastric and breast cancers [96,97]. Qu et al. [77] found a positive correlation between serum exosomal miRNA-665 levels and advanced clinical parameters, including tumor size and patient mortality, suggesting the involvement of serum exosomal miRNA-665 in HCC development. Exosomal miRNA-665 promoted HCC cell proliferation by increasing the phosphorylation of ERK, as demonstrated by western blotting and in vivo experiments. Anti-miRNA-665 has also been shown to be effective in suppressing exosomal miRNA-665 expression.
- Recently, several studies demonstrated that tumor-derived exosomal miRNAs play critical roles in promoting cancer metastasis by regulating target gene expression. Cancer-associated fibroblasts (CAFs), which are activated fibroblasts, are strongly associated with tumor progression and metastasis, and enhance the tumorigenicity of tumor cells via interleukin-6 and interleukin-8 signaling [98,99]. Fang et al. [78] demonstrated that highly metastatic HCC cells located in the metastatic lung niche secrete exosomal miRNA-1247-3p as an onco-miRNA. This secretion leads to the downregulation of β-1,4-galactosyltransferase III and the activation of CAFs via the beta-1-integrin-nuclear factor-κB signaling pathway. Yang et al. [79] reported that hepatoma-derived exosomal miRNA 92a-3p has the potential to promote cancer progression by inducing EMT, which is a critical step in metastasis, downregulation of the tumor suppressor gene PTEN, and activation of the Akt/Snail pathway. High expression of miRNA-92a-3p, facilitated by transcription factors E2F1 and c-Myc, is associated with cancer progression and lung metastasis. This indicates that exosomal miRNA-92a-3p may serve as a predictive marker of HCC metastasis.
- Several studies have examined the association of HCC prognosis with tumor angiogenesis. Previous research has shown that knockdown of SMAD family member 4 (SMAD4) and signal transducer and activator of transcription 6 (STAT6) promotes endothelial cell migration and neovascularization, with SMAD4 and STAT6 potentially serving as inhibitory regulators of angiogenesis [100,101]. Lin et al. [80] demonstrated that exosomal miRNA-210 could be delivered from HCC cells to endothelial cells, thereby enhancing tumor angiogenesis by suppressing the expression of SMAD4 and STAT6. This study is the first to detect communication between HCC and endothelial cells using exosomal miRNA-210.
- Attenuation of junction integrity between endothelial cells promotes tumor metastasis. The endothelial cell junction is composed of the proteins vascular endothelial-cadherin (VE-Cad), catenins, and zonula occludens [102]. Fang et al. [81] demonstrated that exosomal miRNA-103 derived from hepatoma cells increases vascular permeability by reducing endothelial junction integrity and inhibiting the expression of VE-Cad, p120-catenin, and zonula occludens, which promotes HCC metastasis. Hepatoma cell-secreted miRNA-103 has the potential to serve as both a predictive marker and therapeutic target for HCC metastasis.
- Several exosomal miRNAs that function as tumor suppressors in HCC have been reported. Previous studies have shown that miRNA-125b inhibits the EMT, migration, and invasion of HCC cells by downregulating SMAD2/4, SIRT17, SUV39H1, LIN28B, and phosphatidylinositol glycan anchor biosynthesis class F [103-107]. Liu et al. [82] demonstrated that serum exosomal miRNA-125b is a predictive factor associated with time to recurrence (TTR) and overall survival (OS) in patients with HCC. In that study, it was evident that patients with HCC and low miRNA-125b expression had shorter TTR and OS than those with high miRNA-125b expression. Therefore, downregulation of miRNA-125 may be linked to the growth and invasion of HCC cells. According to Shi et al. [83], low expression of serum exosomal miRNA-638 predicts poor prognosis with poor OS in patients with HCC. A negative association was observed between serum exosomal miRNA-638 and clinicopathological tumor characteristics, such as tumor size, vascular invasion, and TNM stage, in patients with HCC. In a previous study, the downregulation of miRNA-638 in HCC facilitated angiogenesis via regulation of vascular endothelial growth factor (VEGF) [108]. Coulouarn et al. [109] reported that miRNA-122 expression tends to be downregulated in HCC, which was linked to worse prognosis; increased tumor growth, invasion, and metastasis; and poor differentiation. In addition, Chen et al. [84] reported that serum miRNA-34a is downregulated in patients with HCC, suggesting its potential as a promising marker for HCC detection and surveillance. Through experiments using a mirRNA-34a mimic, Dang et al. [110] showed that miRNA-34a acts as a tumor suppressor miRNA in HCC, targeting phospho-ERK1/2, phospho-STAT5 signaling, and c-MET. MiRNA-497 is a prognostic marker of HCC. Yan et al. [85] demonstrated that miRNA-497 inhibits VEGF and astrocyte elevated gene-1 (AEG-1) production, which reduces the angiogenesis and metastasis of HCC cells, both in vitro and in vivo.
- 2. Exosomal long noncoding RNAs as prognostic markers
- LncRNA-ATB is an oncogenic factor that induces EMT, tumor invasion, and metastasis by upregulating ZEB1 and ZEB2 [111]. Lee et al. [86] have shown that serum exosomal lncRNA-ATB is a potential prognostic factor for HCC. Elevated levels of exosomal lncRNA-ATB are linked to aggressive tumor features, including TMN stage and portal vein thrombosis, resulting in reduced OS and progression-free survival. In that study, elevated expression of exosomal miRNA-21 was found to be an independent predictor of assessment, and there were no associations between miRNA-21 and lncRNA-ATB. According to Huang et al. [87], lncRNA-PTTG3P may stimulate PI3K/Akt signaling in HCC and upregulate pituitary tumor-transforming 1, which in turn may promote tumor development and metastasis and could be an effective prognostic indicator of HCC.
- Recent studies have investigated the roles of lncRNAs in the treatment of HCC. Although radiofrequency ablation (RFA) is a curative treatment option for patients with early HCC, inadequate RFA has been linked to local recurrence and HCC metastasis [88,112]. According to Ma et al. [88], elevated levels of exosomal lncRNA-ASMTL-AS1 are responsible for the malignant transformation of residual HCC after inadequate RFA treatment. In that study, activation of lncRNA-ASMTL-AS1 by Myc resulted in the downregulation of miRNA-342-3p, exacerbating HCC cell malignancy by activating nemo-like kinase (NLK)/YAP signaling. The authors proposed that lncRNA-ASMTL-AS1 may serve as a novel predictive and therapeutic target for residual HCC after RFA [88].
- In prior research, expression of the LIM kinase (LIMK) family, which is characterized by modulation of the actin cytoskeleton, was associated with metastasis progression [113,114]. LIMK1 was identified as a miRNA-520-3p target. As a tumor suppressor, miRNA-520-3p inhibits HCC progression by downregulation of the LIMK1 axis. Notably, lncRNA H19 accelerates HCC proliferation and metastasis by increasing LIMK1 activation through miRNA-520-3P sequestration [89].
- 3. Exosomal circular RNAs as prognostic markers
- Several exosomal circRNAs have been reported to be associated with target genes and miRNAs. Wang et al. [89] demonstrated that metastasis-related serum exosomal circPTGR1 is associated with advanced tumor clinical stage and poor prognosis via the miRNA-449a/MET pathway. Previous study have shown that overexpression of miRNA-449a, which functions as a tumor suppressor, inhibits tumor cell growth, migration, and invasion [115]. The proto-oncogene MET is one of the targets of miRNA-449a. circPTGR1 may play a crucial role in downregulating the interactions between miRNA-449a and MET, which can disrupt the homeostasis of the tumor microenvironment (TME) and enhance HCC progression. Considering that circPTGR1 is highly expressed in malignant metastatic HCC cells, it may serve as a prognostic and therapeutic marker in patients with HCC.
- Zhang et al. [90] revealed that exosomal circular ubiquitin-like with plant homeodomain and ring finger domain 1 RNA (circUHRF1), generated in large amounts by HCC cells, induces natural killer (NK) cell dysfunction in HCC, thereby assisting with immunosuppression. Thus, it was demonstrated that circUHRF1 may contribute to resistance to anti-PD1 immunotherapy in patients with HCC. Gong et al. [91] showed that the circ-ZEB1.33-miRNA-200a-3p-cyclin dependent kinase 6 (CDK6) regulatory axis plays a role in the reinforcement of proliferation in human HCC; circ-ZEB1.33, which stimulates the growth of human HCC by targeting miRNA-200a-3p and elevating CDK6, was detected in tumors and serum, and its levels may be utilized to predict the outcomes of individual patients.
- Huang et al. [92] demonstrated that exosomal circRNA-100338 promotes HCC metastasis by affecting cell proliferation, angiogenesis, and vessel permeability. Moreover, high expression of exosomal circRNA-100338 may serve as a prognostic factor for lung metastasis and poor survival in patients with HCC who have undergone curative hepatectomy. Exosomal circRNAs have been identified as tumor suppressors in HCC. Chen et al. [65] reported that exosomal circRNA-0051443 inhibited HCC progression by sponging miRNA-331-3p and regulating BCL2 antagonist/killer 1 (BAK1) expression. Previous studies have shown that BAK1 is a target of miRNA-331-3p and a regulator of mitochondria-mediated apoptosis, which is linked to the development of several cancers, including cervical and non-small cell lung cancers [116,117].
Prognostic markers of exosomal noncoding RNAs of hepatocellular carcinoma
- Recently, researchers have explored the use of recipient cell-derived exosomal ncRNAs as therapeutic treatments for HCC (Table 3 [88,90,118-126]). In a previous study, downregulation of exosomal miRNA-122 was linked to HCC development and progression [42]. Lou et al. [118] demonstrated that miR-122 delivered through adipose tissue-derived mesenchymal cell exosomes was transferred to recipient HCC cells, enhancing their sensitivity to systemic chemotherapy, including sorafenib. Similarly, Wang et al. [119] showed that exosomes derived from stellate cells loaded with miRNA-335-5p reduced HCC progression and metastasis and caused tumor shrinkage. Moh-Moh-Aung et al. [120] found a negative correlation between miRNA200b-3p and ETS-related gene (ERG), a gene associated with HCC angiogenesis. Additionally, exosomes from an HCC cell line overexpressing miRNA200b-3p were used to demonstrate the ability of miRNA200b-3p to decrease angiogenesis by reducing ERG expression. Using engineered exosomes designed to selectively bind to HepG2 cells, it was confirmed that upregulated miRNA-26a reduces the migration and proliferation capabilities of the cells [121].
- Efforts are underway to investigate exosomal lncRNAs as potential therapeutic targets for HCC. Analysis of the miRNA-342-3p/NLK/YAP signaling pathway revealed a high level of exosomal ASMTL-AS1 expression in cases where HCC persisted after inadequate treatment with RFA. Consequently, regulating the expression of ASMTL-AS1 through lncRNA may serve as a potential therapeutic strategy for HCC [88].
- Recent studies have indicated that circRNAs are associated with the immunosuppressive TME of HCC. Huang et al. [122] have revealed that circMET is upregulated in HCC tissues via the miRNA-30-5p/Snail/dipeptidyl peptidase 4 (DPP4)/CXCL10 pathway, which further exacerbates the immunosuppressive TME. Moreover, the combination of the DPP4 inhibitor sitagliptin and an anti-PD1 antibody has been shown to elevate the number of CD8+T lymphocytes infiltrating the tissue microarray [122]. Zhang et al. [90] demonstrated a positive correlation between the expression of circUHRF1 in the exosomes of HCC cells and poor clinical outcomes in patients with HCC. In addition, analysis of the molecular mechanisms of circUHRF1 in NK cells showed that a high level of plasma exosomal circUHRF1 was associated with a reduction in the NK cell proportion and tumor infiltration. Modulation of exosomal circUHRF1 demonstrated therapeutic potential in regulating the immunosuppressant TME in HCC cases resistant to anti-PD1 treatment [90].
- Several natural compounds and their derivatives provide innovative possibilities for cancer treatment. Zhou et al. [123] found that HCC proliferation and metastasis are inhibited by gomisin M1 and its analogs, which target TAR-RNA binding protein to control miRNA synthesis, change the levels of a subset of miRNAs (such as miRNA-497-5p, miRNA-146a-5p, and miRNA-10b-5p), and affect pathways relevant to cancer. Tang et al. [124] reported that solamargine substantially reduced the production of mucin short variant S1 (MUC1) protein by upregulating the expression of miRNA-4726-5p, which is regulated by the lncRNAs HOTTIP and TUG1. Their study demonstrated that solamargine inhibited HCC growth and boosted the anticancer effect of sorafenib through the HOTTIP-TUG1/miRNA-4726-5p/MUC1 signaling axis. These findings suggest prospective therapeutic targets and approaches for HCC management. Li et al. [125] found that notoginsenoside R1 inhibits the PI3K/Akt pathway and exhibits anti-hepatoma activity by downregulating miRNA-21. Li et al. [126] demonstrated that oroxin B has efficient anticancer properties by upregulating the production of miRNA-221, which in turn causes the PI3K/Akt signaling pathway to be inactivated and liver cancer cells to undergo apoptosis. These findings reveal the possibility of antitumor effects in HCC. Zhang et al. [127] demonstrated that sanguinarine could stimulate the production of miRNA-16 in HCC cells with either wild-type or mutant p53, but not in cells with p53 eradicated. More notably, sanguinarine may cause cell cycle arrest and apoptosis, inhibiting the proliferation of tumor cells.
- Further research is necessary to assess the role of tumor suppressors in the transport of exosomal lncRNAs and circRNAs. Investigating the correlation between target genes/miRNAs, exosomal lncRNAs, and circRNAs could lead to the development of novel therapeutic targets for the treatment of HCC.
Therapeutic tools of exosomal noncoding RNAs in hepatocellular carcinoma
- This review provides details of exosomal ncRNAs, such as miRNAs, lncRNAs, and circRNAs, their target genes in patients with HCC, and their clinical applications. However, the clinical applications of serum exosomal ncRNAs in HCC are restricted by an inadequate understanding of ncRNA regulatory networks and the complexity of target gene associations. It is critical to establish the precise relationship between exosomal ncRNAs and their target gene networks using extensive and validated prospective data.
- Owing to the abundance of exosomes in biofluids such as ascitic fluid, utilizing exosomal ncRNAs derived from ascitic fluid for the diagnosis and prognostic evaluation of HCC is promising, surpassing the limitations of conventional blood-derived exosomal ncRNAs. Given that radiological modalities have relatively low sensitivity for the early diagnosis of HCC, it would be worthwhile to explore the correlation between radiological imaging markers, exosomal ncRNAs, and the classic biomarker AFP. Exosomal ncRNAs have potential as biomarkers for early diagnosis and prognosis and as therapeutic tools in HCC.
Conclusion
-
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
-
Acknowledgements
The authors are grateful to Hyo Eun Lee, the design director (Institute of Medical Science, Yeungnam University).
-
Funding
This research was supported in part by grants from the Bio & Medical Technology Development Program of the National Research Foundation (NRF) of the Korean government (grant numbers: 2019M3E5D1A02068089 and 2021R1G1A1094767).
-
Author contributions
Conceptualization, Funding acquisition, Investigation: MKK; Data curation, Visualization: JSY, MKK; Writing-original draft: JSY, MKK; Writing-review & editing: JSY, MKK.
Article information
| Cargo | Change | Source of exosome | Target genes/miRNA | Samples | Significance in HCC patients | Reference |
|---|---|---|---|---|---|---|
| Exosomal miRNAs | ||||||
| miRNA-21 | Up | Serum | NA | NC (n=30), CHB (n=30), HCC (n=30) | Potential biomarker for HCC diagnosis | [41] |
| miRNA-21, miRNA-10b, miRNA-122, miRNA-200a | Up | Serum | NA | Rats (n=108) | Diagnostic markers in early stage of HCC with AFP | [42] |
| miRNA-122, miRNA-148a, miRNA-1246 | Up | Serum | NA | NC (n=64), CHB (n=50), LC (n=53), HCC (n=68) | Distinguishable markers of HCC from LC and NC with AFP | [43] |
| miRNA-18a, miRNA-221, miRNA-222, miRNA-224 | Up | Serum | NA | CHB (n=20), LC (n=20), HCC (n=20) | Distinguishable markers of HCC from CHB or LC | [44] |
| miRNA-101, miRNA-106b, miRNA-122, miRNA-195 | Down | |||||
| miRNA-10b-5p | Up | Serum | NA | NC (n=19), mUICC stage IV HCC (n=19) | Potential diagnostic biomarker for early-stage HCC | [45] |
| miRNA-148a | Down | Plasma | NA | NC (n=95), LC (n=96), HCC (n=155) | A novel noninvasive biomarker for diagnosis of HCC | [46] |
| miRNA-155-5p | Up | Serum | PTEN | Paied with HCC tissue and non-cancerous liver tissue (n=28) | Novel target for HCC diagnosis and therapy | [47] |
| Exosomal lncRNAs | ||||||
| lncRNA-HEIH | Up | Serum | NA | CHC (n=35), LC (n=22), HCC (n=10) | Distinguishable marker of HCC from CHC or LC | [48] |
| lncRNA-HULC | Up | Plasma | HULC/miRNA-2052/MET | NC (n=20), HCC (n=30) | Diagnostic and prognostic maker in HCC | [49,50] |
| linc00152 | Up | Serum | EpCAM | NC (n=93), CHB (n=27), LC (n=49), HCC (n=129) | Diagnostic markers in HCC | [51,63] |
| linc00853 | Up | Serum | NA | non-HCC (n=92), HCC (n=90) | Diagnostic markers in HCC | [52] |
| lncRNA-00161 | Up | Serum | NA | NC (n=15), HCC (n=15) | Diagnostic markers in HCC | [53] |
| lncRNA-ENSG00000258332.1, lncRNA-00635 | Up | Serum | NA | NC (n=60), CHB (n=96), | Diagnostic markers in HCC with AFP | [54] |
| LC (n=85), HCC (n=60) | ||||||
| lncRNA-RP11-583F2.2 | Up | Serum | miRNA-1298 | NC (n=18), CHC (n=42), HCC (n=60) | Diagnostic marker in HCC | [55] |
| lncRNA-SNHG1 | Up | Plasma | miRNA-21 | NC (n=50), LC (n=50), HCC (n=72) | Diagnostic marker in HCC | [56] |
| lncRNA-FAL1 | Up | Serum | ZEB1/ceRNA of miRNA-1236 | NC (n=30), HCC (n=30) | Diagnostic marker in HCC | [57] |
| lncRNA-UCA1 | Up | Serum | miRNA-216b, FGFR1/ERK | NC (n=98), HCC (n=98) | Diagnositc/therapeutic marker in HCC | [58,63] |
| lncRNA-MALAT1 | Up | Serum | miRNA-204/SIRT, miRNA-200a | NC (n=93), CHB (n=27), LC (n=49), HCC (n=129) | Diagnostic marker in HCC | [59,60,63] |
| lncRNA-UBE2CP3 | Up | Serum | EMT | NC (n=93), CHB (n=27), LC (n=49), HCC (n=129) | Diagnostic marker in HCC | [61,63] |
| lncRNA-SPRY4-IT1 | Up | Serum | NA | NC (n=93), CHB (n=27), LC (n=49), HCC (n=129) | Diagnostic marker in HCC | [62,63] |
| lncRNA-85 | Up | Plasma | Sponge function of miRNA-324-5p | LC (n=43), HCC (n=112) | Distinguishable marker in AFP-negative HCC from NC and LC | [64] |
| Exosomal circRNAs | ||||||
| circRNA-0051443 | Down | Plasma | BAK1/miRNA-331-3p | NC (n=60), HCC (n=60) | Diagnostic marker in HCC from NC | [65] |
| circRNA-104075 | Down | Serum | miRNA-582-3p/YAP | NC (n=60), HCC (n=10) | Diagnostic marker in HCC from NC | [66] |
| circSMARCA5 | Down | Plasma | NA | NC (n=33), LC (n=31), HCC (n=133) | Diagnostic marker in HCC from NC | [67] |
| circ-CDYL | Up | HCC tissue | Sponge of miRNA-892a, miRNA-328-3P | HCC (n=149) | Diagnostic marker in early-stage HCC | [68] |
miRNA, microRNA; lncRNA, long noncoding RNA; circRNA, circular RNA; NA, not applicable; NC, normal control; CHB, chronic hepatitis B; CHC, chronic hepatitis C; LC, liver cirrhosis; AFP, α-fetoprotein; mUICC, modified Union for International Cancer Control; HULC, highly upregulated in liver cancer; EpCAM, epithelial cell adhesion molecule; MALAT1, metastasis-associated lung adenocarcinoma transcript 1; YAP, yes-associated protein; circ‐CDYL, circ‐chromodomain Y like.
| Cargo | Change | Source of exosome | Target genes or pathway/miRNA | Samples | Significance in HCC patients | Reference |
|---|---|---|---|---|---|---|
| Exosomal miRNAs | ||||||
| miRNA-21 | Up | Serum | NA | NC (n=30), CHB (n=30), HCC (n=30) | HCC progression | [41] |
| miRNA-665 | Up | Serum | MARK/ERK | NC (n=10), HCC (n=30) | HCC progression | [77] |
| miRNA-1247-3p | Up | Serum | B4GALT3 | HCC (n=85) | HCC lung metastasis | [78] |
| miRNA-92a-3p | Up | Hepatoma cell | E2F1 and c-Myc | HCC without metastasis (n=21) | HCC metastasis | [79] |
| HCC with metastasis (n=21) | ||||||
| miRNA-210 | Up | Serum | SMAD4, STAT6 | NC (n=60), HCC (n=104) | HCC angiogenesis | [80] |
| miRNA-103 | Up | Hepatoma cell | NA | HCC (n=85) | HCC angiogenesis and metastasis | [81] |
| miRNA-125b | Down | Serum | NA | Cohort 1: CHB (n=30), LC (n=30), HCC (n=30) | HCC suppression | [82] |
| Cohort 2: HCC (n=128) | ||||||
| miRNA-638 | Down | Serum | NA | HCC (n=126) | HCC suppression | [83] |
| miRNA-122 | Up | Whole blood | NA | HCC (n=177) | TACE refractoriness in HCC patients | [93] |
| miRNA-34a | Down | Serum | NA | NC (n=60), HCC (n=60) | TNM stage, lymph node, vascular metastasis of HCC | [84] |
| miRNA-497 | Down | Serum | VEGFA, AEG-1 | Recombinant lentivirus LV-miR-497 vs. control LV-miR-NC | Suppress angiogenesis and metastasis of HCC | [85] |
| Exosomal lncRNAs | ||||||
| lncRNA-ATB | Up | Serum | NA | HCC (n=79) | HCC progression | [86] |
| lncRNA-ASMTL-AS1 | Up | Serum | miRNA-342-3p/NLK/YAP | HCC (n=70) | HCC progression after insufficient RFA | [88] |
| lncRNA-PTTG3P | Up | Tissue | PTTG3P, PI3K/Akt | Two HCC cohort (n=46, 90) | HCC prognosis | [87] |
| Exosomal circRNAs | ||||||
| circPTGR1 | Up | Serum | miRNA-449a/MET | NC (n=41), HCC (n=71) | HCC metastasis | [89] |
| circUHRF1 | Up | Plasma | IFN-γ, TNF-α | HCC (n=240) | Resistance to anti-PD1 immunotherapy | [90] |
| circZEB1.33 | Up | Serum/tissue | miRNA200a-3P/CDK6 | NC (n=30), HCC (n=60) | HCC proliferation | [91] |
| circRNA-100338 | Up | Serum | NA | HCC (n=39) | HCC angiogenesis and metastasis | [92] |
| circRNA-0051443 | Down | Plasma | BAK1 | NC (n=60), HCC (n=60) | HCC suppression | [65] |
miRNA, microRNA; lncRNA, long noncoding RNA; circRNA, circular RNA; NA, not applicable; NC, normal control; CHB, chronic hepatitis B; CHC, chronic hepatitis C; LC, liver cirrhosis; RFA, radiofrequency ablation; MARK, map/microtubule affinity-regulating kinase; ERK, extracellular signal-regulated kinase; SMAD4, SMAD family member 4; STAT6, signal transducer and activator of transcription 6; VEGFA, vascular endothelial growth factor A; AEG-1, astrocyte elevated gene-1; TACE, transcatheter arterial chemoembolization; lncRNA-ATB, long noncoding RNA activated by transforming growth factor beta; ASMTL-AS1, antisense MTMR12 antisense RNA 1; NLK, nemo-like kinase; YAP, yes-associated protein; PTTG3P, pituitary tumor-transforming 3, pseudogene; PI3K/Akt, phosphoinositide 3-kinase/protein kinase B; MET, mesenchymal-epithelial transition; IFN, interferon; TNF, tumor necrosis factor.
| Cargo | Change | Source of exosome | Target genes/miRNA | Samples | Significance | Reference |
|---|---|---|---|---|---|---|
| Exosomal miRNAs | ||||||
| miRNA-335-5p | Down | CAFs | - | Several HCC cells | Extracellualar vesicles (EV)-miRNA-335-5p can decrease cancer growth and invasion in vitro and in vivo | [119] |
| miRNA-200b-3p | Down | Cells | ERG | NC (n=40), HCC (n=40) | Angiogenesis of HCC | [120] |
| miRNA-26a | Down | Cells | Apo-A1 | HepG2 cells | Decreased the rates of cell migration and proliferation: regulation of cell cycle | [121] |
| miRNA-122 | Down | Cells | Adipose tissue-derived MSC | HepG2 cells | Promote chemosensitivity of HCC cells | [117] |
| Exosomal lncRNAs | ||||||
| lncRNA-ASMTL-AS1 | Up | Serum | miRNA-342-3p/NLK/YAP | HCC (n=70) | HCC progression after insufficient RFA | [118] |
| Exosomal circRNAs | ||||||
| circMET | Up | Cells | miRNA-30-5p | HCC (n=209) | Anti-PD1 therapy resistance in HCC | [122] |
| circUHRF1 | Down | Cells | miRNA-449c-5p | HCC (n=240) | Anti-PD1 therapy resistance in HCC | [90] |
| Natural compounds | ||||||
| Gomisin M1 | TRBP/miRNA-497-5p, miRNA-126a-5p, miRNA-10b-5p | Inhibit the HCC proliferation and metastasis | [123] | |||
| Solarmargine | HOTTIP-TUG1/miRNA-4726-5p/MUC1 | Inhibit the HCC proliferation and enhance the anticancer effect of sorafenib | [124] | |||
| Notoginsenoside R1 | miRNA-21 | Antitumor effect of HCC | [125] | |||
| Oroxin B | miRNA-221, PTEN/PI3K/Akt | Induce apoptosis in HCC | [126] | |||
| Sanguinarine | P53, miRNA 16-2 | Induce cell cycle arrest and apoptosis in HCC | [127] |
miRNA, microRNA; lncRNA, long noncoding RNA; circRNA, circular RNA; CAFs, cancer-associated fibroblasts; NC, normal control; RFA, radiofrequency ablation; EV, extracellular vesicles; HCC, hepatocellular carcinoma; Apo-A1, apolipoprotein A1; MSC, mesenchymal stem cells; ASMTL-AS1, antisense MTMR12 antisense RNA 1; NLK, nemo-like kinase; YAP, yes-associated protein; PD1, programmed cell death protein 1; TRBP, TAR RNA binding protein; HOTTIP-TUG1, HOXA transcript at the distal tip-taurine upregulated gene 1; MUC1, mucin 1; PI3K/Akt, phosphoinositide 3-kinase/protein kinase B.
- 1. Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet 2022;400:1345–62.ArticlePubMed
- 2. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17–48.ArticlePubMedPDF
- 3. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–2.ArticlePubMedPMC
- 4. Yang H, Woo HY, Lee SK, Han JW, Jang B, Nam HC, et al. A comparative study of sorafenib and metronomic chemotherapy for Barcelona Clinic Liver Cancer-stage C hepatocellular carcinoma with poor liver function. Clin Mol Hepatol 2017;23:128–37.ArticlePubMedPMCPDF
- 5. Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 2008;100:698–711.ArticlePubMed
- 6. Schwartz M, Roayaie S, Konstadoulakis M. Strategies for the management of hepatocellular carcinoma. Nat Clin Pract Oncol 2007;4:424–32.ArticlePubMedPDF
- 7. Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003;38:1449–57.ArticlePubMed
- 8. Chang Y, Kim JI, Lee B, Kim SG, Jung MJ, Kim YS, et al. Clinical application of ultrasonography-guided percutaneous liver biopsy and its safety over 18 years. Clin Mol Hepatol 2020;26:318–27.ArticlePubMedPMCPDF
- 9. Kim BR, Lee JM, Lee DH, Yoon JH, Hur BY, Suh KS, et al. Diagnostic performance of gadoxetic acid-enhanced liver MR imaging versus multidetector CT in the detection of dysplastic nodules and early hepatocellular carcinoma. Radiology 2017;285:134–46.ArticlePubMed
- 10. Wang G, Zhu S, Li X. Comparison of values of CT and MRI imaging in the diagnosis of hepatocellular carcinoma and analysis of prognostic factors. Oncol Lett 2019;17:1184–8.ArticlePubMedPMC
- 11. El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118–27.ArticlePubMed
- 12. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004;116:281–97.ArticlePubMed
- 13. Calin GA, Croce CM. MicroRNA-cancer connection: the beginning of a new tale. Cancer Res 2006;66:7390–4.ArticlePubMedPDF
- 14. Wong CM, Tsang FH, Ng IO. Non-coding RNAs in hepatocellular carcinoma: molecular functions and pathological implications. Nat Rev Gastroenterol Hepatol 2018;15:137–51.ArticlePubMedPDF
- 15. van Malenstein H, van Pelt J, Verslype C. Molecular classification of hepatocellular carcinoma anno 2011. Eur J Cancer 2011;47:1789–97.ArticlePubMed
- 16. Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov 2017;16:203–22.ArticlePubMedPDF
- 17. Han TS, Ban HS, Hur K, Cho HS. The epigenetic regulation of HCC metastasis. Int J Mol Sci 2018;19:3978.ArticlePubMedPMC
- 18. Bae HJ, Jung KH, Eun JW, Shen Q, Kim HS, Park SJ, et al. MicroRNA-221 governs tumor suppressor HDAC6 to potentiate malignant progression of liver cancer. J Hepatol 2015;63:408–19.ArticlePubMed
- 19. Ruan K, Fang X, Ouyang G. MicroRNAs: novel regulators in the hallmarks of human cancer. Cancer Lett 2009;285:116–26.ArticlePubMed
- 20. Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med 2014;20:460–9.ArticlePubMed
- 21. Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem 2012;81:145–66.ArticlePubMedPMC
- 22. Kartha RV, Subramanian S. Competing endogenous RNAs (ceRNAs): new entrants to the intricacies of gene regulation. Front Genet 2014;5:8.ArticlePubMedPMC
- 23. Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature 2014;505:344–52.ArticlePubMedPMCPDF
- 24. Wang X, Sun W, Shen W, Xia M, Chen C, Xiang D, et al. Long non-coding RNA DILC regulates liver cancer stem cells via IL-6/STAT3 axis. J Hepatol 2016;64:1283–94.ArticlePubMed
- 25. Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell 2016;29:452–63.ArticlePubMedPMC
- 26. Li X, Yang L, Chen LL. The biogenesis, functions, and challenges of circular RNAs. Mol Cell 2018;71:428–42.ArticlePubMed
- 27. Greene J, Baird AM, Brady L, Lim M, Gray SG, McDermott R, et al. Circular RNAs: biogenesis, function and role in human diseases. Front Mol Biosci 2017;4:38.ArticlePubMedPMC
- 28. Ebbesen KK, Hansen TB, Kjems J. Insights into circular RNA biology. RNA Biol 2017;14:1035–45.ArticlePubMedPMC
- 29. Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 2008;10:1470–6.ArticlePubMedPMCPDF
- 30. Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A 2004;101:13368–73.ArticlePubMedPMC
- 31. Lässer C, Alikhani VS, Ekström K, Eldh M, Paredes PT, Bossios A, et al. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med 2011;9:9.ArticlePubMedPMC
- 32. Ruivo CF, Adem B, Silva M, Melo SA. The biology of cancer exosomes: insights and new perspectives. Cancer Res 2017;77:6480–8.ArticlePubMedPDF
- 33. Zaborowski MP, Balaj L, Breakefield XO, Lai CP. Extracellular vesicles: composition, biological relevance, and methods of study. Bioscience 2015;65:783–97.ArticlePubMedPMC
- 34. Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol 2015;77:13–27.ArticlePubMed
- 35. Xiao D, Ohlendorf J, Chen Y, Taylor DD, Rai SN, Waigel S, et al. Identifying mRNA, microRNA and protein profiles of melanoma exosomes. PLoS One 2012;7:e46874.ArticlePubMedPMC
- 36. Pant S, Hilton H, Burczynski ME. The multifaceted exosome: biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem Pharmacol 2012;83:1484–94.ArticlePubMedPMC
- 37. Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007;9:654–9.ArticlePubMedPDF
- 38. Lemoinne S, Thabut D, Housset C, Moreau R, Valla D, Boulanger CM, et al. The emerging roles of microvesicles in liver diseases. Nat Rev Gastroenterol Hepatol 2014;11:350–61.ArticlePubMedPDF
- 39. Nicholas J. A new diagnostic tool with the potential to predict tumor metastasis. J Natl Cancer Inst 2013;105:371–2.ArticlePubMed
- 40. Yuen MF, Cheng CC, Lauder IJ, Lam SK, Ooi CG, Lai CL. Early detection of hepatocellular carcinoma increases the chance of treatment: Hong Kong experience. Hepatology 2000;31:330–5.ArticlePubMed
- 41. Wang H, Hou L, Li A, Duan Y, Gao H, Song X. Expression of serum exosomal microRNA-21 in human hepatocellular carcinoma. Biomed Res Int 2014;2014:864894.ArticlePubMedPMCPDF
- 42. Liu WH, Ren LN, Wang X, Wang T, Zhang N, Gao Y, et al. Combination of exosomes and circulating microRNAs may serve as a promising tumor marker complementary to alpha-fetoprotein for early-stage hepatocellular carcinoma diagnosis in rats. J Cancer Res Clin Oncol 2015;141:1767–78.ArticlePubMedPDF
- 43. Wang Y, Zhang C, Zhang P, Guo G, Jiang T, Zhao X, et al. Serum exosomal microRNAs combined with alpha-fetoprotein as diagnostic markers of hepatocellular carcinoma. Cancer Med 2018;7:1670–9.ArticlePubMedPMC
- 44. Sohn W, Kim J, Kang SH, Yang SR, Cho JY, Cho HC, et al. Serum exosomal microRNAs as novel biomarkers for hepatocellular carcinoma. Exp Mol Med 2015;47:e184.ArticlePubMedPMCPDF
- 45. Cho HJ, Eun JW, Baek GO, Seo CW, Ahn HR, Kim SS, et al. Serum exosomal microRNA, miR-10b-5p, as a potential diagnostic biomarker for early-stage hepatocellular carcinoma. J Clin Med 2020;9:281.ArticlePubMedPMC
- 46. Han J, Li J, Qian Y, Liu W, Liang J, Huang Z, et al. Identification of plasma miR-148a as a noninvasive biomarker for hepatocellular carcinoma. Clin Res Hepatol Gastroenterol 2019;43:585–93.ArticlePubMed
- 47. Fu X, Wen H, Jing L, Yang Y, Wang W, Liang X, et al. MicroRNA-155-5p promotes hepatocellular carcinoma progression by suppressing PTEN through the PI3K/Akt pathway. Cancer Sci 2017;108:620–31.ArticlePubMedPMC
- 48. Zhang C, Yang X, Qi Q, Gao Y, Wei Q, Han S. lncRNA-HEIH in serum and exosomes as a potential biomarker in the HCV-related hepatocellular carcinoma. Cancer Biomark 2018;21:651–9.ArticlePubMed
- 49. Xie H, Ma H, Zhou D. Plasma HULC as a promising novel biomarker for the detection of hepatocellular carcinoma. Biomed Res Int 2013;2013:136106.ArticlePubMedPMCPDF
- 50. Wang Y, Chen F, Zhao M, Yang Z, Li J, Zhang S, et al. The long noncoding RNA HULC promotes liver cancer by increasing the expression of the HMGA2 oncogene via sequestration of the microRNA-186. J Biol Chem 2017;292:15395–407.ArticlePubMedPMC
- 51. Ji J, Tang J, Deng L, Xie Y, Jiang R, Li G, et al. LINC00152 promotes proliferation in hepatocellular carcinoma by targeting EpCAM via the mTOR signaling pathway. Oncotarget 2015;6:42813–24.ArticlePubMedPMC
- 52. Kim SS, Baek GO, Ahn HR, Sung S, Seo CW, Cho HJ, et al. Serum small extracellular vesicle-derived LINC00853 as a novel diagnostic marker for early hepatocellular carcinoma. Mol Oncol 2020;14:2646–59.ArticlePubMedPMCPDF
- 53. Sun L, Su Y, Liu X, Xu M, Chen X, Zhu Y, et al. Serum and exosome long non coding RNAs as potential biomarkers for hepatocellular carcinoma. J Cancer 2018;9:2631–9.ArticlePubMedPMC
- 54. Xu H, Chen Y, Dong X, Wang X. Serum exosomal long noncoding RNAs ENSG00000258332.1 and LINC00635 for the diagnosis and prognosis of hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev 2018;27:710–6.ArticlePubMedPDF
- 55. Matboli M, Labib ME, Nasser HE, El-Tawdi AH, Habib EK, Ali-Labib R. Exosomal miR-1298 and lncRNA-RP11-583F2.2 expression in hepato-cellular carcinoma. Curr Genomics 2020;21:46–55.ArticlePubMedPMC
- 56. Gao S, Xu X, Wang Y, Zhang W, Wang X. Diagnostic utility of plasma lncRNA small nucleolar RNA host gene 1 in patients with hepatocellular carcinoma. Mol Med Rep 2018;18:3305–13.ArticlePubMedPMC
- 57. Li B, Mao R, Liu C, Zhang W, Tang Y, Guo Z. LncRNA FAL1 promotes cell proliferation and migration by acting as a CeRNA of miR-1236 in hepatocellular carcinoma cells. Life Sci 2018;197:122–9.ArticlePubMed
- 58. Wang F, Ying HQ, He BS, Pan YQ, Deng QW, Sun HL, et al. Upregulated lncRNA-UCA1 contributes to progression of hepatocellular carcinoma through inhibition of miR-216b and activation of FGFR1/ERK signaling pathway. Oncotarget 2015;6:7899–917.ArticlePubMedPMC
- 59. Hou Z, Xu X, Zhou L, Fu X, Tao S, Zhou J, et al. The long non-coding RNA MALAT1 promotes the migration and invasion of hepatocellular carcinoma by sponging miR-204 and releasing SIRT1. Tumour Biol 2017;39:1010428317718135.ArticlePubMedPDF
- 60. Zhao ZB, Chen F, Bai XF. Long noncoding RNA MALAT1 regulates hepatocellular carcinoma growth under hypoxia via sponging microRNA-200a. Yonsei Med J 2019;60:727–34.ArticlePubMedPMCPDF
- 61. Cao SW, Huang JL, Chen J, Hu YW, Hu XM, Ren TY, et al. Long non-coding RNA UBE2CP3 promotes tumor metastasis by inducing epithelial-mesenchymal transition in hepatocellular carcinoma. Oncotarget 2017;8:65370–85.ArticlePubMedPMC
- 62. Jing W, Gao S, Zhu M, Luo P, Jing X, Chai H, et al. Potential diagnostic value of lncRNA SPRY4-IT1 in hepatocellular carcinoma. Oncol Rep 2016;36:1085–92.ArticlePubMed
- 63. Huang J, Zheng Y, Xiao X, Liu C, Lin J, Zheng S, et al. A circulating long noncoding RNA panel serves as a diagnostic marker for hepatocellular carcinoma. Dis Markers 2020;2020:5417598.ArticlePubMedPMCPDF
- 64. Huang X, Sun L, Wen S, Deng D, Wan F, He X, et al. RNA sequencing of plasma exosomes revealed novel functional long noncoding RNAs in hepatocellular carcinoma. Cancer Sci 2020;111:3338–49.ArticlePubMedPMCPDF
- 65. Chen W, Quan Y, Fan S, Wang H, Liang J, Huang L, et al. Exosome-transmitted circular RNA hsa_circ_0051443 suppresses hepatocellular carcinoma progression. Cancer Lett 2020;475:119–28.ArticlePubMed
- 66. Zhang X, Xu Y, Qian Z, Zheng W, Wu Q, Chen Y, et al. circRNA_104075 stimulates YAP-dependent tumorigenesis through the regulation of HNF4a and may serve as a diagnostic marker in hepatocellular carcinoma. Cell Death Dis 2018;9:1091.ArticlePubMedPMCPDF
- 67. Li Z, Zhou Y, Yang G, He S, Qiu X, Zhang L, et al. Using circular RNA SMARCA5 as a potential novel biomarker for hepatocellular carcinoma. Clin Chim Acta 2019;492:37–44.ArticlePubMed
- 68. Wei Y, Chen X, Liang C, Ling Y, Yang X, Ye X, et al. A noncoding regulatory RNAs network driven by circ-CDYL acts specifically in the early stages hepatocellular carcinoma. Hepatology 2020;71:130–47.ArticlePubMedPDF
- 69. Tomimaru Y, Eguchi H, Nagano H, Wada H, Kobayashi S, Marubashi S, et al. Circulating microRNA-21 as a novel biomarker for hepatocellular carcinoma. J Hepatol 2012;56:167–75.ArticlePubMed
- 70. Connolly E, Melegari M, Landgraf P, Tchaikovskaya T, Tennant BC, Slagle BL, et al. Elevated expression of the miR-17-92 polycistron and miR-21 in hepadnavirus-associated hepatocellular carcinoma contributes to the malignant phenotype. Am J Pathol 2008;173:856–64.ArticlePubMedPMC
- 71. Su H, Yang JR, Xu T, Huang J, Xu L, Yuan Y, et al. MicroRNA-101, down-regulated in hepatocellular carcinoma, promotes apoptosis and suppresses tumorigenicity. Cancer Res 2009;69:1135–42.ArticlePubMedPDF
- 72. Wong QW, Lung RW, Law PT, Lai PB, Chan KY, To KF, et al. MicroRNA-223 is commonly repressed in hepatocellular carcinoma and potentiates expression of Stathmin1. Gastroenterology 2008;135:257–69.ArticlePubMed
- 73. Rybak-Wolf A, Stottmeister C, Glažar P, Jens M, Pino N, Giusti S, et al. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol Cell 2015;58:870–85.ArticlePubMed
- 74. Li W, Dong X, He C, Tan G, Li Z, Zhai B, et al. LncRNA SNHG1 contributes to sorafenib resistance by activating the Akt pathway and is positively regulated by miR-21 in hepatocellular carcinoma cells. J Exp Clin Cancer Res 2019;38:183.ArticlePubMedPMCPDF
- 75. Gao R, Cai C, Gan J, Yang X, Shuang Z, Liu M, et al. miR-1236 down-regulates alpha-fetoprotein, thus causing PTEN accumulation, which inhibits the PI3K/Akt pathway and malignant phenotype in hepatoma cells. Oncotarget 2015;6:6014–28.ArticlePubMedPMC
- 76. Cao L, Xie B, Yang X, Liang H, Jiang X, Zhang D, et al. MiR-324-5p suppresses hepatocellular carcinoma cell invasion by counteracting ECM degradation through post-transcriptionally downregulating ETS1 and SP1. PLoS One 2015;10:e0133074.ArticlePubMedPMC
- 77. Qu Z, Wu J, Wu J, Ji A, Qiang G, Jiang Y, et al. Exosomal miR-665 as a novel minimally invasive biomarker for hepatocellular carcinoma diagnosis and prognosis. Oncotarget 2017;8:80666–78.ArticlePubMedPMC
- 78. Fang T, Lv H, Lv G, Li T, Wang C, Han Q, et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat Commun 2018;9:191.ArticlePubMedPMCPDF
- 79. Yang B, Feng X, Liu H, Tong R, Wu J, Li C, et al. High-metastatic cancer cells derived exosomal miR92a-3p promotes epithelial-mesenchymal transition and metastasis of low-metastatic cancer cells by regulating PTEN/Akt pathway in hepatocellular carcinoma. Oncogene 2020;39:6529–43.ArticlePubMedPMCPDF
- 80. Lin XJ, Fang JH, Yang XJ, Zhang C, Yuan Y, Zheng L, et al. Hepatocellular carcinoma cell-secreted exosomal microRNA-210 promotes angiogenesis in vitro and in vivo. Mol Ther Nucleic Acids 2018;11:243–52.ArticlePubMedPMC
- 81. Fang JH, Zhang ZJ, Shang LR, Luo YW, Lin YF, Yuan Y, et al. Hepatoma cell-secreted exosomal microRNA-103 increases vascular permeability and promotes metastasis by targeting junction proteins. Hepatology 2018;68:1459–75.ArticlePubMedPDF
- 82. Liu W, Hu J, Zhou K, Chen F, Wang Z, Liao B, et al. Serum exosomal miR-125b is a novel prognostic marker for hepatocellular carcinoma. Onco Targets Ther 2017;10:3843–51.ArticlePubMedPMCPDF
- 83. Shi M, Jiang Y, Yang L, Yan S, Wang YG, Lu XJ. Decreased levels of serum exosomal miR-638 predict poor prognosis in hepatocellular carcinoma. J Cell Biochem 2018;119:4711–6.ArticlePubMedPDF
- 84. Chen S, Mao Y, Chen W, Liu C, Wu H, Zhang J, et al. Serum exosomal miR-34a as a potential biomarker for the diagnosis and prognostic of hepatocellular carcinoma. J Cancer 2022;13:1410–7.ArticlePubMedPMC
- 85. Yan JJ, Zhang YN, Liao JZ, Ke KP, Chang Y, Li PY, et al. MiR-497 suppresses angiogenesis and metastasis of hepatocellular carcinoma by inhibiting VEGFA and AEG-1. Oncotarget 2015;6:29527–42.ArticlePubMedPMC
- 86. Lee YR, Kim G, Tak WY, Jang SY, Kweon YO, Park JG, et al. Circulating exosomal noncoding RNAs as prognostic biomarkers in human hepatocellular carcinoma. Int J Cancer 2019;144:1444–52.ArticlePubMedPDF
- 87. Huang JL, Cao SW, Ou QS, Yang B, Zheng SH, Tang J, et al. The long non-coding RNA PTTG3P promotes cell growth and metastasis via up-regulating PTTG1 and activating PI3K/AKT signaling in hepatocellular carcinoma. Mol Cancer 2018;17:93.ArticlePubMedPMCPDF
- 88. Ma D, Gao X, Liu Z, Lu X, Ju H, Zhang N. Exosome-transferred long non-coding RNA ASMTL-AS1 contributes to malignant phenotypes in residual hepatocellular carcinoma after insufficient radiofrequency ablation. Cell Prolif 2020;53:e12795.ArticlePubMedPMCPDF
- 89. Wang G, Liu W, Zou Y, Wang G, Deng Y, Luo J, et al. Three isoforms of exosomal circPTGR1 promote hepatocellular carcinoma metastasis via the miR449a-MET pathway. EBioMedicine 2019;40:432–45.ArticlePubMedPMC
- 90. Zhang PF, Gao C, Huang XY, Lu JC, Guo XJ, Shi GM, et al. Cancer cell-derived exosomal circUHRF1 induces natural killer cell exhaustion and may cause resistance to anti-PD1 therapy in hepatocellular carcinoma. Mol Cancer 2020;19:110.ArticlePubMedPMCPDF
- 91. Gong Y, Mao J, Wu D, Wang X, Li L, Zhu L, et al. Circ-ZEB1.33 promotes the proliferation of human HCC by sponging miR-200a-3p and upregulating CDK6. Cancer Cell Int 2018;18:116.ArticlePubMedPMCPDF
- 92. Huang XY, Huang ZL, Huang J, Xu B, Huang XY, Xu YH, et al. Exosomal circRNA-100338 promotes hepatocellular carcinoma metastasis via enhancing invasiveness and angiogenesis. J Exp Clin Cancer Res 2020;39:20.ArticlePubMedPMCPDF
- 93. Kim SS, Nam JS, Cho HJ, Won JH, Kim JW, Ji JH, et al. Plasma micoRNA-122 as a predictive marker for treatment response following transarterial chemoembolization in patients with hepatocellular carcinoma. J Gastroenterol Hepatol 2017;32:199–207.ArticlePubMedPDF
- 94. Han Y, Wu Z, Wu T, Huang Y, Cheng Z, Li X, et al. Tumor-suppressive function of long noncoding RNA MALAT1 in glioma cells by downregulation of MMP2 and inactivation of ERK/MAPK signaling. Cell Death Dis 2016;7:e2123.ArticlePubMedPMCPDF
- 95. Carlo-Stella C, Locatelli SL, Giacomini A, Cleris L, Saba E, Righi M, et al. Sorafenib inhibits lymphoma xenografts by targeting MAPK/ERK and AKT pathways in tumor and vascular cells. PLoS One 2013;8:e61603.ArticlePubMedPMC
- 96. Qu JL, Qu XJ, Zhao MF, Teng YE, Zhang Y, Hou KZ, et al. Gastric cancer exosomes promote tumour cell proliferation through PI3K/Akt and MAPK/ERK activation. Dig Liver Dis 2009;41:875–80.ArticlePubMed
- 97. Wang W, Zou L, Zhou D, Zhou Z, Tang F, Xu Z, et al. Overexpression of ubiquitin carboxyl terminal hydrolase-L1 enhances multidrug resistance and invasion/metastasis in breast cancer by activating the MAPK/Erk signaling pathway. Mol Carcinog 2016;55:1329–42.ArticlePubMedPDF
- 98. Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature 2004;432:332–7.ArticlePubMedPMCPDF
- 99. Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005;121:335–48.ArticlePubMed
- 100. Schwarte-Waldhoff I, Volpert OV, Bouck NP, Sipos B, Hahn SA, Klein-Scory S, et al. Smad4/DPC4-mediated tumor suppression through suppression of angiogenesis. Proc Natl Acad Sci U S A 2000;97:9624–9.ArticlePubMedPMC
- 101. Nishimura Y, Nitto T, Inoue T, Node K. IL-13 attenuates vascular tube formation via JAK2-STAT6 pathway. Circ J 2008;72:469–75.ArticlePubMed
- 102. Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev 2004;84:869–901.ArticlePubMed
- 103. Liang L, Wong CM, Ying Q, Fan DN, Huang S, Ding J, et al. MicroRNA-125b suppressesed human liver cancer cell proliferation and metastasis by directly targeting oncogene LIN28B2. Hepatology 2010;52:1731–40.ArticlePubMed
- 104. Zhou JN, Zeng Q, Wang HY, Zhang B, Li ST, Nan X, et al. MicroRNA-125b attenuates epithelial-mesenchymal transitions and targets stem-like liver cancer cells through small mothers against decapentaplegic 2 and 4. Hepatology 2015;62:801–15.ArticlePubMed
- 105. Kim JK, Noh JH, Jung KH, Eun JW, Bae HJ, Kim MG, et al. Sirtuin7 oncogenic potential in human hepatocellular carcinoma and its regulation by the tumor suppressors MiR-125a-5p and MiR-125b. Hepatology 2013;57:1055–67.ArticlePubMedPDF
- 106. Fan DN, Tsang FH, Tam AH, Au SL, Wong CC, Wei L, et al. Histone lysine methyltransferase, suppressor of variegation 3-9 homolog 1, promotes hepatocellular carcinoma progression and is negatively regulated by microRNA-125b. Hepatology 2013;57:637–47.ArticlePubMed
- 107. Alpini G, Glaser SS, Zhang JP, Francis H, Han Y, Gong J, et al. Regulation of placenta growth factor by microRNA-125b in hepatocellular cancer. J Hepatol 2011;55:1339–45.ArticlePubMedPMC
- 108. Cheng J, Chen Y, Zhao P, Liu X, Dong J, Li J, et al. Downregulation of miRNA-638 promotes angiogenesis and growth of hepatocellular carcinoma by targeting VEGF. Oncotarget 2016;7:30702–11.ArticlePubMedPMC
- 109. Coulouarn C, Factor VM, Andersen JB, Durkin ME, Thorgeirsson SS. Loss of miR-122 expression in liver cancer correlates with suppression of the hepatic phenotype and gain of metastatic properties. Oncogene 2009;28:3526–36.ArticlePubMedPMCPDF
- 110. Dang Y, Luo D, Rong M, Chen G. Underexpression of miR-34a in hepatocellular carcinoma and its contribution towards enhancement of proliferating inhibitory effects of agents targeting c-MET. PLoS One 2013;8:e61054.ArticlePubMedPMC
- 111. Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ, Tao QF, et al. A long noncoding RNA activated by TGF-β promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell 2014;25:666–81.ArticlePubMed
- 112. Teng W, Liu KW, Lin CC, Jeng WJ, Chen WT, Sheen IS, et al. Insufficient ablative margin determined by early computed tomography may predict the recurrence of hepatocellular carcinoma after radiofrequency ablation. Liver Cancer 2015;4:26–38.ArticlePubMedPMCPDF
- 113. Bernard O. Lim kinases, regulators of actin dynamics. Int J Biochem Cell Biol 2007;39:1071–6.ArticlePubMed
- 114. Li R, Doherty J, Antonipillai J, Chen S, Devlin M, Visser K, et al. LIM kinase inhibition reduces breast cancer growth and invasiveness but systemic inhibition does not reduce metastasis in mice. Clin Exp Metastasis 2013;30:483–95.ArticlePubMedPDF
- 115. You J, Zhang Y, Li Y, Fang N, Liu B, Zu L, et al. MiR-449a suppresses cell invasion by inhibiting MAP2K1 in non-small cell lung cancer. Am J Cancer Res 2015;5:2730–44.PubMedPMC
- 116. Wang YD, Cai N, Wu XL, Cao HZ, Xie LL, Zheng PS. OCT4 promotes tumorigenesis and inhibits apoptosis of cervical cancer cells by miR-125b/BAK1 pathway. Cell Death Dis 2013;4:e760.ArticlePubMedPMCPDF
- 117. Gu XY, Wang J, Luo YZ, Du Q, Li RR, Shi H, et al. Down-regulation of miR-150 induces cell proliferation inhibition and apoptosis in non-small-cell lung cancer by targeting BAK1 in vitro. Tumour Biol 2014;35:5287–93.ArticlePubMedPDF
- 118. Lou G, Song X, Yang F, Wu S, Wang J, Chen Z, et al. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol 2015;8:122.ArticlePubMedPMC
- 119. Wang F, Li L, Piontek K, Sakaguchi M, Selaru FM. Exosome miR-335 as a novel therapeutic strategy in hepatocellular carcinoma. Hepatology 2018;67:940–54.ArticlePubMedPMCPDF
- 120. Moh-Moh-Aung A, Fujisawa M, Ito S, Katayama H, Ohara T, Ota Y, et al. Decreased miR-200b-3p in cancer cells leads to angiogenesis in HCC by enhancing endothelial ERG expression. Sci Rep 2020;10:10418.ArticlePubMedPMCPDF
- 121. Liang G, Kan S, Zhu Y, Feng S, Feng W, Gao S. Engineered exosome-mediated delivery of functionally active miR-26a and its enhanced suppression effect in HepG2 cells. Int J Nanomedicine 2018;13:585–99.ArticlePubMedPMCPDF
- 122. Huang XY, Zhang PF, Wei CY, Peng R, Lu JC, Gao C, et al. Circular RNA circMET drives immunosuppression and anti-PD1 therapy resistance in hepatocellular carcinoma via the miR-30-5p/snail/DPP4 axis. Mol Cancer 2020;19:92.ArticlePubMedPMCPDF
- 123. Zhou Z, Li Y, Ma X, Cao B, Peng T, Sheng Y, et al. Identification of a novel TAR RNA-binding protein 2 modulator with potential therapeutic activity against hepatocellular carcinoma. J Med Chem 2021;64:7404–21.ArticlePubMed
- 124. Tang Q, Li X, Chen Y, Long S, Yu Y, Sheng H, et al. Solamargine inhibits the growth of hepatocellular carcinoma and enhances the anticancer effect of sorafenib by regulating HOTTIP-TUG1/miR-4726-5p/MUC1 pathway. Mol Carcinog 2022;61:417–32.ArticlePubMedPMCPDF
- 125. Li Y, Li Z, Jia Y, Ding B, Yu J. In vitro anti-hepatoma activities of notoginsenoside R1 through downregulation of tumor promoter miR-21. Dig Dis Sci 2020;65:1364–75.ArticlePubMedPDF
- 126. Li N, Men W, Zheng Y, Wang H, Meng X. Oroxin B induces apoptosis by down-regulating microRNA-221 resulting in the inactivation of the PTEN/PI3K/AKT pathway in liver cancer. Molecules 2019;24:4384.ArticlePubMedPMC
- 127. Zhang B, Wang X, Deng J, Zheng H, Liu W, Chen S, et al. p53-dependent upregulation of miR-16-2 by sanguinarine induces cell cycle arrest and apoptosis in hepatocellular carcinoma. Cancer Lett 2019;459:50–8.ArticlePubMed
References
Figure & Data
References
Citations


 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine PubReader
PubReader ePub Link
ePub Link Cite
Cite