PubMed Central, CAS, DOAJ, KCI

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Volume 41(1); 2024 > Article
-
Case report
DaVinci SP-based simultaneous bilateral partial nephrectomy from the midline transperitoneal approach: a case report -
Young Hwii Ko
 , Jong Gyun Ha
, Jong Gyun Ha , Jae Yoon Jang
, Jae Yoon Jang , Yeung Uk Kim
, Yeung Uk Kim
-
Journal of Yeungnam Medical Science 2024;41(1):48-52.
DOI: https://doi.org/10.12701/jyms.2023.01032
Published online: January 4, 2024
Department of Urology, Yeungnam University College of Medicine, Daegu, Korea
- Corresponding author: Young Hwii Ko, MD, PhD Department of Urology, Yeungnam University College of Medicine, 170 Hyeonchung-ro, Nam-gu, Daegu 42415, Korea Tel: +82-53-620-3694 • Fax: +82-53-627-5535 • E-mail: urokyh@naver.com
Copyright © 2024 Yeungnam University College of Medicine, Yeungnam University Institute of Medical Science
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 809 Views
- 37 Download
Abstract
- While simultaneous bilateral partial nephrectomy with a conventional multiport robot has been consistently reported since the 2010s, the introduction of the DaVinci SP system (Intuitive Surgical, Sunnyvale, CA, USA) could provide a novel way to perform surgery on bilateral kidneys while innovatively reducing the number of incisions. In our first report worldwide, the patient with bilateral small renal mass (2.0 cm for the left and 1.5 cm for the right side) and preoperative normal renal function was placed in the lateral decubitus position on an inverted bed. After tilting the bed to be as horizontal as possible, a 4-cm incision was made in the lower part of the umbilicus for the floating trocar technique. The partial nephrectomy was performed reliably as with the conventional transperitoneal approach, and then the patient could be repositioned to the contralateral side for the same procedure, maintaining all trocars. Total operation time (skin to skin), total console time, and the left- and right-side warm ischemic times were 260, 164, 27, and 23 minutes, respectively, without applying the early declamping technique. The estimated blood loss was 200 mL. The serum creatinine right after the operation, on the first day, 3 days, and 90 days after surgery were 0.92, 0.77, 0.79, and 0.81 mg/dL, respectively. For 90 days after the procedure, no complications or radiologic recurrence were observed. Further clinical studies will reveal the advantages of using the DaVinci SP device for this procedure over traditional multiport surgery, maximizing the benefit of a single port-based approach.
- The minimally invasive approach to intraabdominal surgery in urology has been revolutionized by introducing the DaVinci SP (Intuitive Surgical, Sunnyvale, CA, USA), whose benefits can be maximized in bilateral renal masses, where conventional multiport requires too many holes to insert trocars for the robotic instrument. Bilateral renal tumors account for approximately 3% of renal tumors [1], and a simultaneous bilateral partial nephrectomy with a conventional multiport robot has been consistently reported since the 2010s. However, applying the DaVinci SP device by the transperitoneal approach through a median small incision for the floating trocar technique could provide an alternative surgical option that allows a bilateral approach to the kidney under a single anesthesia session. Here, we present the surgical technique and literature review.
Introduction
- Ethical statements: This study was approved by the Institutional Review Board (IRB) of Yeungnam University Hospital (IRB No: YUMC-2023-08-019). Written informed consent was obtained from the patient for the publication of this report including all clinical images.
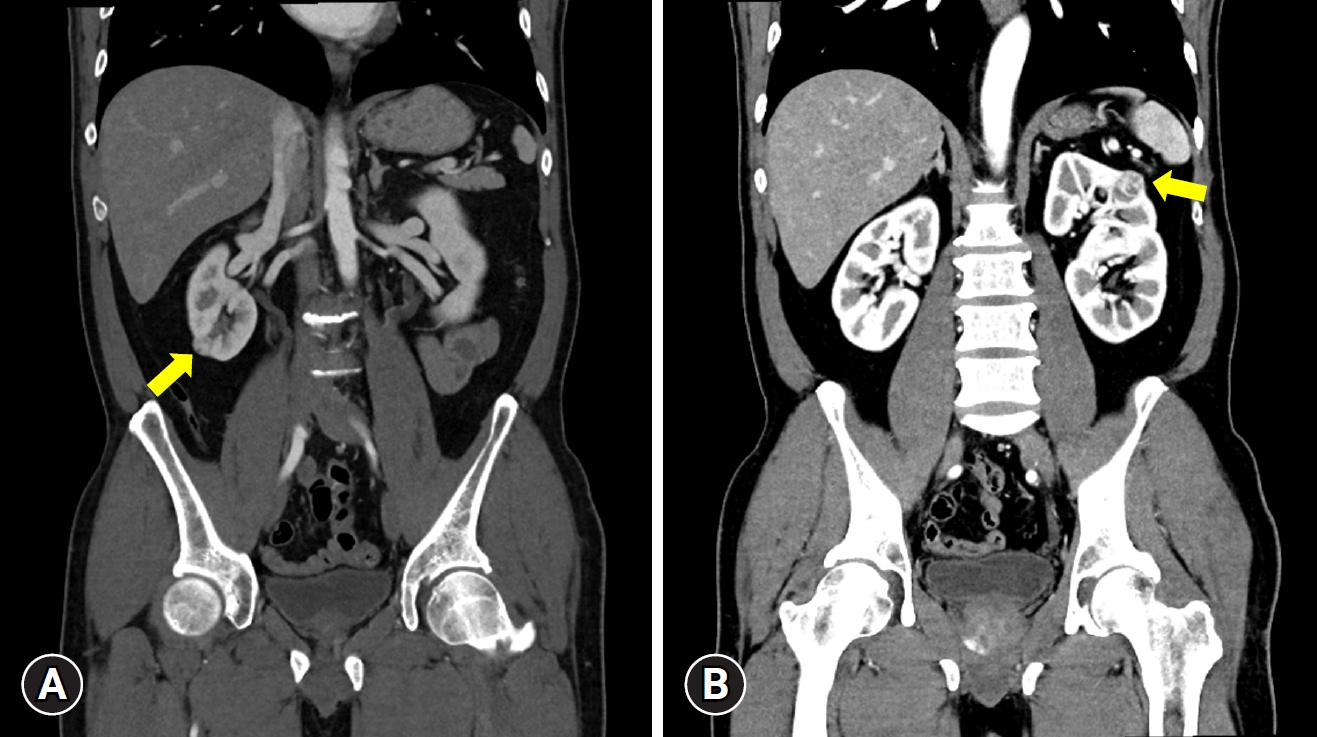
- A 52-year-old man presents to the outpatient urology department with an incidentally discovered bilateral kidney mass by ultrasound. On the abdominal computed tomography (CT), the masses measured 1.5 cm on the right and 2.0 cm on the left. The RENAL (radius, exophytic/endophytic, nearness to collecting system or sinus, anterior/posterior, and location relative to polar lines) nephrometry score was 5 for both sides the PADUA (preoperative aspects and dimensions used for an anatomical) score was 7 for the left and 8 for the right sides (Fig. 1). The patient had no previous history of surgery or trauma and no history of comorbidities or medications. Preoperatively, serum creatinine and estimated glomerular filtration rate (eGFR) were 0.81 mg/dL and 86.7 mL/min/1.73 m2, respectively. All other preoperative laboratory findings were within normal limits. His body mass index was 24.3 kg/m2. Given the relatively small size of the masses, a preoperative biopsy was not performed.
- Given the larger mass in the left kidney and two renal arteries on the CT image, a left-side procedure was attempted first. The patient was placed on the right-side lateral decubitus position on an inverted bed. After tilting the bed to be as horizontal as possible, a 4 cm incision was made in the lower part of the umbilicus. Then, an SP Access Kit (manufactured by Intuitive Surgical) was installed for the floating trocar technique. An additional port for the assistant manipulating the laparoscopic device was made on the midline above 5 cm from the umbilicus, with a blind supporting the peritoneum with fingers through the previously made midline incision (Fig. 2A).
- The surgical procedure involved robotic instruments, specifically monopolar curved scissors, Fenestrated bipolar forceps, Cadiere forceps, and needle drivers. Robotic partial nephrectomy was performed using the same procedure as the usual transperitoneal approach. In brief, the double renal artery was isolated, and two separate vessel loops were placed around them. The location and depth of the tumor were confirmed by intraoperative ultrasound, then the surface of the kidney was scored using a monopolar curved scissor. After clamping the arteries, indocyanine green was administrated to confirm complete ischemic status utilizing the firefly function. After careful resection of the tumor surrounding the pseudo capsule of the tumor, the baseline bleeding was secured with 15 cm of 3-0 observable suture, then resected renal parenchyma were closed with interrupted fashion utilizing the same suture material embedded with a large-sized surgical clip at the distal end. The procedures were performed without applying the early-declamping technique to minimize potential bleeding. The specimen was removed through the midline port without a laparoscopic sac device. The robot was undocked, and an Ioban 2 antimicrobial incise drape (3M Company, St. Paul, MS, USA) was applied, maintaining all the trocars inserted into the peritoneum (Fig. 3A). The patient’s position was changed to the left decubitus, and the robot was re-docked to the same port (Fig. 3B). The operation on the right kidney was performed similarly, with the drain installed through the assist port site in the center, just like the already inserted left (Fig. 2A).
- Total operation time (skin to skin) was 260 minutes, including the console time of 164 minutes. Among the console time, the left-side procedure was taken 109 minutes, with a warm ischemic time (WIT) of 27 minutes. The console and WIT were 55 minutes and 23 minutes for the right-side procedure, respectively. The estimated blood loss was 200 mL. The patient started a diet the next day and was discharged from the hospital on the third day according to the terms of his insurance (Fig. 2B). No perioperative complication, including blood transfusion, occurred.
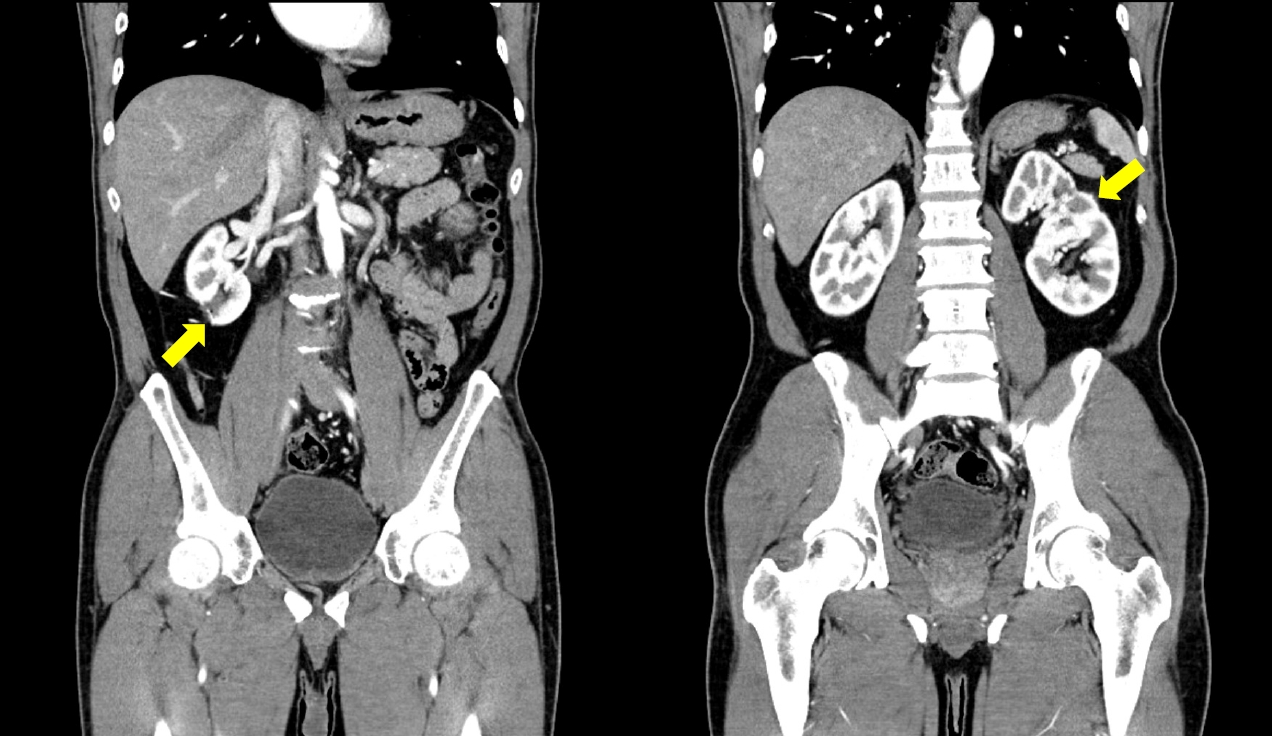
- Pathologic reports confirm a clear-cell type of renal cell carcinoma with margin negativity for both sides. Fuhrman grade was 2/4 for both sides. The serum creatinine right after the operation, on the first day, the day of discharge, and 90 days after surgery were 0.92, 0.77, 0.79, and 0.81 mg/dL, respectively. There were no adverse events such as hematuria, flank pain, or readmission requiring further management for 90 days after the procedure, and no abnormalities such as surgical site recurrence, anastomotic leakage, or local inflammation were observed on the abdominal CT at 3 months (Fig. 4).
Case
- In the case of simultaneously detected kidney cancer, the kidney cancer on one side is often too large to be preserved. Though it could be found in the form of synchronous metastasis on the contralateral side from the large-sized tumor [2], kidney preservation is increasingly possible if detected early with the ubiquity of imaging diagnostics [3]. Nevertheless, the optimal treatment strategies for patients with synchronous bilateral renal tumors have not been established yet.
- Given the potential risk of high-grade kidney cancer and the patient has already clinically progressed to synchronous metastasis, systemic treatment may be considered, but surgical removal is the most commonly attempted treatment for kidney cancer detected in the absence of distant metastases. Previously, the main treatment options for bilateral renal tumors were unilateral nephrectomy combined with partial resection for the contralateral side or bilateral partial nephrectomy [4-6]. Because bilateral nephrectomy could have a devastating impact on quality of life and loss of renal function could potentially shorten a patient's life expectancy, the renal-sparing approach has become the preferred option when feasible. Non-surgical removal options such as cryoablation, radiofrequency ablation, or irreversible electroporation may be performed as alternative methods of kidney preservation but have not been reported to perform as well as partial nephrectomy in long-term follow-up.
- Regarding the timing of partial nephrectomy for bilateral masses, there has been controversy over whether it should be performed simultaneously or sequentially. In the absence of guideline statements, partial nephrectomy for bilateral kidney cancer has been tried as a staged operation in many cases and has been accepted traditionally as the standard approach. Lowrance et al. [6] reported that the staged procedure could minimize the decline in renal function, and patients could avoid dialysis treatment. However, the number of reports demonstrating no significant elevated risk in the requirement for dialysis after the simultaneous procedure has increased. In a report from the Mayo Clinic of 75 simultaneous bilateral cases, including most cases by open series performed from 1974 to 2013, eight patients suffered acute renal failure during the perioperative period, but none progressed to dialysis [3]. The eGFR values exhibited a median decrease of –19 mL/min/1.73 m2 before and after surgery; therefore, the authors recommended the staged procedure for patients with preoperative impaired renal function. From the retrospective data accumulated over a decade (2009–2018), Di Maida et al. [7] compared the perioperative outcomes from the simultaneous procedure with the staged one. Among 41 patients included, a simultaneous approach was chosen in 17 patients (42%). Patients treated with a staged strategy showed significantly higher median cumulative operative time (310 minutes vs. 240 minutes, p=0.01), WIC (18 minutes vs. 10 minutes, p=0.03), and length of stay (10 days vs. 6 days, p=0.01) than patients receiving simultaneous surgery. No significant differences were found according to the median change of eGFR from the baseline to 3 months and disease-free survival in patients treated with simultaneous versus staged surgery.
- Although retrospective, there are a growing number of reports of reliable simultaneous bilateral partial nephrectomy in the robotic era. Otoshi et al. [8] first reported their simultaneous robotic partial nephrectomy series for eight patients, with the tumors’ median size of 1.4 cm (range, 0.9–9.0 cm). The eGFR 30 days after surgery decreased slightly compared to before but recovered to the preoperative level with no significant differences. In the most extensive patient report to date, Gallo et al. [9] performed simultaneous robotic partial nephrectomy in 27 patients, with a median operative time of 250 minutes and a median WIT of 15 minutes. However, the complications were reported in seven patients (25.9%), mainly represented by Clavien-Dindo grade II events (six blood transfusions), and positive surgical margins were assessed in two of them (3.7%).
- The unique advantage of robotic partial nephrectomy is that it can be performed through a single port compared to conventional multiport robots. In a systematic review of five comparative articles comparing the perioperative outcomes from the conventional multiport performing robot-assisted partial nephrectomy published, a recent systemic review demonstrated similar effectiveness and safety, with a marginally shorter length of hospital stay and less blood loss by a single port-based approach [10]. Simultaneous partial nephrectomy via transperitoneal midline approach could be a novel surgical category that maximizes the benefits of these previously proposed robotic single port surgeries and the technological advances of the DaVinci SP device, maintaining acceptable surgical, oncological, and functional outcomes. Further clinical studies will reveal the advantages of using this device for this procedure over traditional multiport surgery, maximizing the benefit of a single port-based approach.
Discussion
-
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
-
Funding
None.
-
Author contributions
Conceptualization: all authors; Data curation: YHK, JGH, JYJ; Formal analysis, Supervision: YUK; Methodology: JYJ, YUK; Investigation: YHK, JGH; Software: JYJ; Writing-original draft: YHK; Writing-review & editing: YHK.
Article information


- 1. Patel AR, Lee BH, Campbell SC, Zhou M, Fergany AF. Bilateral synchronous sporadic renal tumors: pathologic concordance and clinical implications. Urology 2011;78:1095–9.ArticlePubMed
- 2. Phelan MW. Small renal mass with contralateral large renal mass: remove large renal mass first in staged fashion. Pro. J Urol 2012;188:18–9.ArticlePubMed
- 3. Mason RJ, Atwell T, Lohse C, Bhindi B, Schmit G, Schmitz J, et al. Synchronous nephron-sparing approaches for bilateral renal masses: peri-operative and renal functional outcomes. BJU Int 2018;122:243–8.ArticlePubMedPDF
- 4. Simmons MN, Brandina R, Hernandez AV, Gill IS. Surgical management of bilateral synchronous kidney tumors: functional and oncological outcomes. J Urol 2010;184:865–72.ArticlePubMed
- 5. Wang B, Gong H, Zhang X, Li H, Ma X, Song E, et al. Bilateral synchronous sporadic renal cell carcinoma: retroperitoneoscopic strategies and intermediate outcomes of 60 patients. PLoS One 2016;11:e0154578.ArticlePubMedPMC
- 6. Lowrance WT, Yee DS, Maschino AC, Cronin AM, Bernstein M, Thompson RH, et al. Developments in the surgical management of sporadic synchronous bilateral renal tumours. BJU Int 2010;105:1093–7.ArticlePubMed
- 7. Di Maida F, Grosso AA, Sforza S, Mari A, Lambertini L, Nardoni S, et al. Surgical management of synchronous, bilateral renal masses: a 1-decade referral center experience. Eur Urol Focus 2022;8:1309–17.ArticlePubMed
- 8. Otoshi T, Yamasaki T, Hirayama Y, Uchida J. Pilot experience of simultaneous robotic-assisted partial nephrectomy for bilateral renal tumors-single center analysis. Asian J Endosc Surg 2021;14:57–62.ArticlePubMedPDF
- 9. Gallo F, Sforza S, Luciani L, Mattevi D, Barzaghi P, Mari A, et al. Simultaneous robotic partial nephrectomy for bilateral renal masses. World J Urol 2022;40:1005–10.ArticlePubMedPDF
- 10. Li KP, Chen SY, Wang CY, Yang L. Perioperative and oncologic outcomes of single-port versus conventional robotic-assisted partial nephrectomy: an evidence-based analysis of comparative outcomes. J Robot Surg 2023;17:765–77.ArticlePubMedPDF
References
Figure & Data
References
Citations


 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine PubReader
PubReader ePub Link
ePub Link Cite
Cite





