PubMed Central, CAS, DOAJ, KCI

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Volume 40(4); 2023 > Article
-
Focused Review article
State-of-the-art update for diagnosing diabetic foot osteomyelitis: a narrative review -
Inha Woo1
 , Seung Jae Cho1
, Seung Jae Cho1 , Chul Hyun Park2
, Chul Hyun Park2
-
Journal of Yeungnam Medical Science 2023;40(4):321-327.
DOI: https://doi.org/10.12701/jyms.2023.00976
Published online: October 12, 2023
1Department of Orthopaedic Surgery, Yeungnam University Hospital, Daegu, Korea
2Department of Orthopaedic Surgery, Yeungnam University College of Medicine, Daegu, Korea
- Corresponding author: Chul Hyun Park, MD, PhD Department of Orthopaedic Surgery, Yeungnam University College of Medicine, 170 Hyeonchung-ro, Nam-gu, Daegu 42415, Korea Tel: +82-53-620-3640 • Fax: +82-53-628-4020 • E-mail: chpark77@naver.com
Copyright © 2023 Yeungnam University College of Medicine, Yeungnam University Institute of Medical Science
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Recently, the International Working Group on the Diabetic Foot and the Infectious Diseases Society of America divided diabetic foot disease into diabetic foot infection (DFI) and diabetic foot osteomyelitis (DFO). DFI is usually diagnosed clinically, while numerous methods exist to diagnose DFO. In this narrative review, the authors aim to summarize the updated data on the diagnosis of DFO. An extensive literature search using “diabetic foot [MeSH]” and “osteomyelitis [MeSH]” or “diagnosis” was performed using PubMed and Google Scholar in July 2023. The possibility of DFO is based on inflammatory clinical signs, including the probe-to-bone (PTB) test. Elevated inflammatory biochemical markers, especially erythrocyte sedimentation rate, are beneficial. Distinguishing abnormal findings of plain radiographs is also a first-line approach. Moreover, sophisticated modalities, including magnetic resonance imaging and nuclear medicine imaging, are helpful if doubt remains after a first-line diagnosis. Transcutaneous bone biopsy, which does not pass through the wound, is necessary to avoid contaminating the sample. This review focuses on the current diagnostic techniques for DFOs with an emphasis on the updates. To obtain the correct therapeutic results, selecting a proper option is necessary. Based on these numerous diagnosis modalities and indications, the proper choice of diagnostic tool can have favorable treatment outcomes.
- Diabetes mellitus (DM) is a devastating disease that affects multiple organs, including the heart, kidneys, and nerves [1,2]. Diabetic foot is a common complication in approximately 6.3% of patients with DM [3]. It starts almost as a small wound in the skin and soft tissues as a form of diabetic foot infection (DFI). However, it eventually invades the underlying bone and causes diabetic foot osteomyelitis (DFO). Although 20% of outpatient patients with DFI are associated with DFO afterward, it is worth mentioning that the harm caused by neglecting DFO diagnosis deteriorates anatomical structures and the overall quality of life. Approximately 20% of outpatient cases of DFI result in osteomyelitis [4]. Following DFI, DFO is not a simple bone inflammatory disease but a complicated disease associated with infection, peripheral arterial disease, and peripheral neuropathy, a leading cause of lower extremity amputation [5,6].
- Since 1999, the International Working Group on the Diabetic Foot (IWGDF) and the Infectious Diseases Society of America (IDSA) have regularly proposed evidence-based guidelines and a consensus scheme for diagnosing diabetes-related foot diseases [7-11]. According to the classical IWGDF/IDSA classification, the occurrence of DFO is classified at 3 or 4 and considered severe [8]. In 2020, the updated version of the guidelines and recommendations addressed DFI and DFO separately [10]. According to this new classification, DFO was distinguished by adding the letter “(O)” after the conventional classification system.
- Diagnosis methods for DFI are generally made based on clinical findings. Indicators include erythema, induration, tenderness, warmth, and drainage [12]. However, there are numerous methods for diagnosing DFO, and few studies have addressed the 2023 IWGDF guidelines so far. This narrative review aims to summarize the updated data diagnosing DFO based on the new guidelines.
Introduction
- An extensive literature search using “diabetic foot [MeSH]” and “osteomyelitis [MeSH]” or “diagnosis” was conducted using PubMed and Google Scholar in July 2023. Only English-language studies containing clinical research were included. The authors also consulted experts outside the group to identify the desired system. Data were collected independently by the authors and discussed for inclusion in this review. Disagreements between authors were also hashed out until a consensus was reached.
- 1. Can diabetic foot osteomyelitis be diagnosed clinically?
- Although a definite diagnosis of DFO requires positive results from histology and cultures of bone specimens, it is not always necessary [13]. The initial diagnosis should be based on clinical signs. Osteomyelitis may be suspected when an ulcer fails to heal for more than 6 weeks despite appropriate wound care, adequate offloading, or adequate blood supply [4,9]. Other clinical elements of suspected DFO include diabetic foot ulcers that are large (i.e., >2 cm), deep (i.e., >3 mm), have an inflammatory toe (“sausage toe”), present synovial fluid drainage, and are located over a bony prominence [8,10,14]. As such, clinicians must assess potential risk factors associated with the onset of DFO, such as an overlying bony prominence, extension to bone or joint, and recurrent or multiple wounds. Of note, acute Charcot foot should be ruled out in cases of inflammatory symptoms such as redness, warmth, tenderness, or local swelling, especially when located at the midfoot and without any wound [15].
- Foot ulcers may not be evaluated because of invisible underlying structures beneath the open wound. Because of the presence of callus or necrotic tissue, thorough debridement at presentation will aid in a more accurate evaluation. If the bone is exposed, there is a high probability of osteomyelitis [16]. The new 2023 IWGDF guidelines emphasized the importance of identifying at-risk factors such as the loss of protective sensation and peripheral artery disease [11]. Therefore, a new risk stratification system and corresponding foot screening frequencies were proposed. Early detection of at-risk lesions became important to prevent progression to DFO, the most severe form of diabetes-related foot disease. However, merely diagnosing DFO based on clinical manifestations does not exclude the presence of osteomyelitis. Other methodologies which will be described later should be combined.
- 2. Is the probe-to-bone (PTB) test still important?
- One of the detection tools for DFO is the probe-to-bone (PTB), which was first introduced in 1995 [17]. It was originally performed with a sterile, blunt, and 14 cm stainless steel eye probe. Striking a bone with a probe indicates the likelihood of osteomyelitis in addition to bone or joint space infection. Normally, the test is conducted by inserting a sterile probe into the open wound and exploring it [18]. If the probe reaches the bony surface, the test is considered positive. This simple test yielded a sensitivity of 66%, a specificity of 85%, and a positive predictive value of 89%. The subsequent study, which evaluated a prospective study of 1,666 patients with diabetic foot and compared histologic results, suggested a sensitivity of 87%, a specificity of 91%, a positive predictive value of 57%, and a negative predictive value of 98% [14,19]. Lam et al. [20] reported that the pooled sensitivity and specificity of the PTB test were 87% and 83%, respectively. Meanwhile, the positive and negative predictive values were 98% and 79%, respectively. Recently, the 2023 IWGDF guidelines still suggested that a positive PTB test combined with abnormalities on plain radiographs and high levels of serum markers of inflammation may support the diagnosis [11].
- 3. Which biochemical markers can predict diabetic foot osteomyelitis?
- Blood tests, including the white blood cell (WBC) count, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and procalcitonin, are commonly associated with DFO. Among these, ESR is the most useful marker; a high level (usually defined as >70 mm/hr) of its value increases the likelihood of future osteomyelitis [15]. The values of WBC, CRP, and procalcitonin return to normal approximately 3 weeks after the start of treatment, while the value of ESR stays high only in the case of DFO [21]. Its value has a high specificity, while its sensitivity was only 28% [22]. Among other markers, WBC and CRP levels are elevated in both soft tissue and bone infections. Differentiating the origin of infections based on these two markers is not useful [13]. Vangaveti et al. [23] showed that procalcitonin is a useful diagnostic test for DFO and differentiates it from cellulitis. From a case-control study, remarkably higher serum procalcitonin level was noted in patients with DFO (as the experimental group) than those with DFI (as the control group). Its sensitivity was 79% compared with 50%, 63%, and 66% for adiponectin, osteoprotegerin, and osteopontin.
- The 2023 IWGDF guidelines still recognized the importance of ESR, CRP, and procalcitonin. However, normal findings of these values do not exclude foot infections; when in doubt, additional radiologic evaluation is recommended [11]. On the other hand, Caruso et al. [24] studied the correlation between a level of parathyroid hormone (PTH) and DFO. The authors hypothesized that the high bone turnover caused by osteomyelitis may affect PTH levels and reported that PTH levels were lower in diabetic patients without osteomyelitis.
- 4. What is the value of plain radiographs in diagnosing diabetic foot osteomyelitis?
- The plain radiograph, characterized by its cost-effectiveness, expeditiousness, and safety, remains readily accessible worldwide. The sensitivity of plain radiographs is lower than that of other imaging modalities; bony abnormalities can be visualized on plain radiographs at least 2 to 4 weeks after the onset of bone infection [25]. Typical radiographic findings include cortical disruption, periosteal elevation, a sequestrum, or gross destruction of cortical bone. Confounding factors such as neuroarthropathy (Charcot arthropathy), history of orthopedic surgery, underlying soft tissue or bone disease, and trauma may make the diagnosis suspect [26,27]. Combining plain radiographs with PTB results in a sensitivity of 88.6%, specificity of 66.7%, positive predictive value of 91.2%, and negative predictive value of 60% [28].
- 5. Is magnetic resonance imaging mandatory for diagnosing diabetic foot osteomyelitis?
- In addition to plain radiographs, magnetic resonance imaging (MRI) can be considered a potential tool for evaluating the severity and extent of bone and soft tissue involvement [29]. MRI has high sensitivity and specificity (90% and 83%, respectively) in diagnosing osteomyelitis [30].
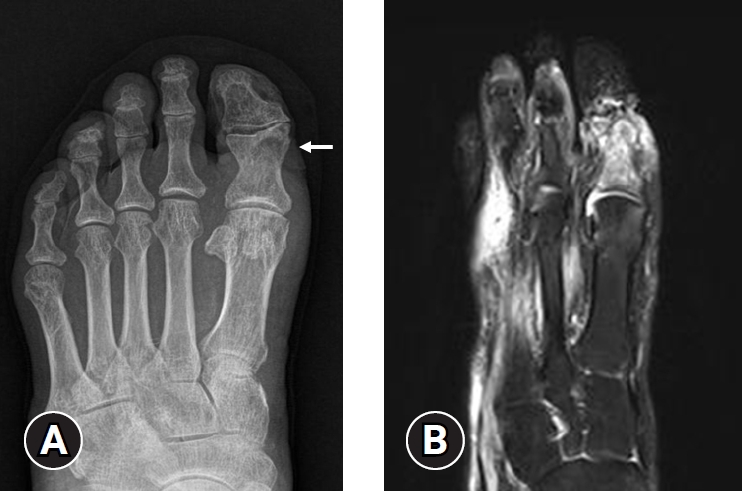
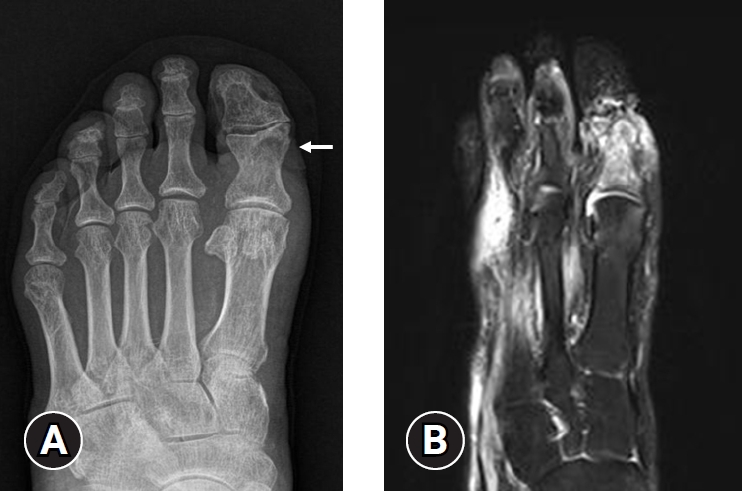
- In 2023, the IWGDF created a new guideline for acute Charcot neuro-osteoarthropathy (CNO). CNO is a sterile inflammatory process in individuals with neuropathy that injures bones, joints, and soft tissues. If not properly treated, it can lead to progressive fractures and dislocations, resulting in a deformed foot [31]. Therefore, distinguishing CNO from typical DFO is critical for clinicians. MRI can differentiate the infected arthropathy from the noninfected arthropathy. The typical MRI findings of DFO include cortical disruption, adjacent soft tissue and bone edema, and sinus tract formation. In contrast, the findings of CNO are predominant midfoot involvement (especially periarticular or subchondral lesions), cyst-like cortical fragmentation, joint deformity or subluxation, and relatively intact overlying skin [15,30,32,33] (Fig. 1).
- Many studies have already shown that MRI has the best diagnostic accuracy and is useful for evaluating the extension and depth of DFO. Nevertheless, it can be challenging to differentiate DFO and bone marrow edema [34]. DFO is bright on short tau inversion recovery (STIR) and T2-weighted (T2W) and confluent hypointense in T1-weighted (T1W) images. In contrast, bone marrow edema is also bright in T2W images, but the T1W image has an intermediate to decreased reticulated hazy intensity [35]. Bone marrow edema may be related to inflammation, infection, tumor, and trauma. La Fontaine et al. [32] reported that in 17 out of 58 patients (29.3%), the impression of DFO based on MRI findings was inconsistent with actual bone biopsy results. Therefore, an integrated approach with clinical findings, MRI results, and bone biopsy (if possible) is crucial for diagnosing DFO accurately, making MRI a gold standard diagnosis but not a vital modality for diagnosing DFO.
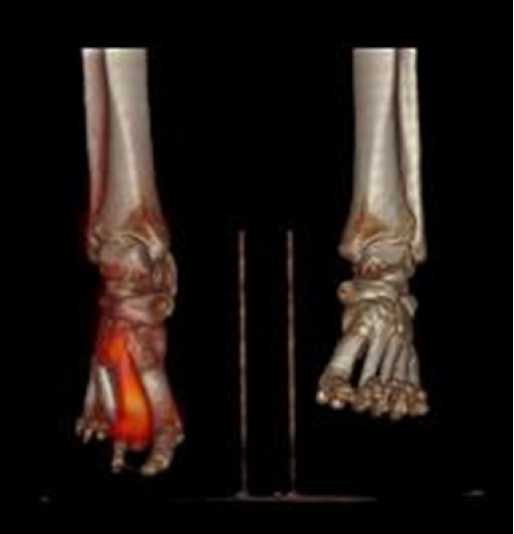
- 6. Which nuclear medicine imaging techniques can help in diagnosing diabetic foot osteomyelitis?
- In cases where bone biopsy is not performed, MRI is the first modality of choice for diagnosing DFO. However, other nuclear medicine imaging modalities, including WBC scintigraphy, 3-phase bone scan, and fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT), can be considered, especially if MRI is contraindicated [8,36]. Among these multiple nuclear medicine modalities, a comprehensive understanding of each tool and proper choice is required.
- Low specificity in distinguishing between soft tissue and bone infection is the common disadvantage of WBC scintigraphy. Moreover, as MRI techniques advanced, the role of WBC scintigraphy became limited. However, it is still sensitive, especially at the earliest stage of bone infection and at follow-up. Low specificity in distinguishing between soft tissue and bone infection is a common limitation [37].
- The technetium (99mTc) 3-phase bone scan plays an important role in distinguishing DFI and DFO. In DFI, tracer activity increases in early phases but is normal in delayed phases. On the contrary, DFO presents increased activities in both early and delayed phases [35].
- Nawaz et al. [38] compared the diagnostic performances between FDG-PET/CT, MRI, and plain radiographs. The researchers concluded that FDG-PET/CT is a highly specific modality and should be considered a useful complementary imaging modality to MRI. WBC scintigraphy can be combined with single-photon emission CT or CT [30]. Uptake is clearly delineated with bone on CT images. This hybrid technique of these two modalities plays an important role in differentiating superficial DFI from DFO. However, false-negative results may be the limitation of this imaging modality during antibiotic treatment or in the presence of underlying severe vascular disease. The sensitivity and specificity of this modality then range from 75% to 100% and from 67% to 100%, respectively [39-42]. Lauri et al. [43] strongly recommended using WBC scintigraphy in suspected pedal osteomyelitis. On the other hand, FDG-PET/CT can show focal or diffuse uptake when osteomyelitis is suspected. A systemic review and meta-analysis suggested that FDG-PET/CT has the highest diagnostic accuracy for confirming or excluding the diagnosis of chronic osteomyelitis [44]. In a meta-analysis published in 2013, the pooled sensitivity and specificity of this modality is 74% and 91%, respectively [45] (Fig. 2).
- 7. When and what type of biopsy is needed?
- The definite diagnosis for DFO can only be made with bone biopsy. It usually provides histopathologic and microbiologic findings [36]. A negative histopathologic bone biopsy can accurately exclude the diagnosis of DFO [46]. Cecilia-Matilla et al. [47] conducted an observational prospective study of 165 patients with diabetic foot ulcers and found four histopathologic types of DFO: acute osteomyelitis, chronic osteomyelitis, chronic acute osteomyelitis, and fibrosis according to bone necrosis, remodeling, bone marrow fibrosis, and periosteal fibrosis. The histologic criteria of DFO include bone erosion, bone marrow edema, fibrosis, necrosis, and the presence of inflammatory cells [14].
- On the other hand, microbiologic results from bone biopsy provide causative pathogen and its susceptible antibiotic information [13]. The 2023 IWGDF guidelines recommended a curettage or biopsy, not a swab, to diagnose osteomyelitis. The most common causative organisms can be diverse, but Staphylococcus aureus is predominant in most cases [11]. In interpreting causative pathogens (i.e., Staphylococcus epidermidis, Corynebacterium spp., Cutibacterium acnes), bacteria from the normal skin flora should be excluded.
- The correct technique of bone biopsy is associated with meaningful results. Superficial swabbing causes low sensitivity, and the concordance rate between bacteria from bone biopsy and superficial swab culture was only 38% [48]. A recent study confirmed that transcutaneous bone biopsy, which does not traverse the wound, is necessary to avoid contaminating the sample [49]. Choosing an appropriate time for bone biopsy is also important. The previous guidelines [8,36] suggested that biopsy should be considered during surgical drainage when the situation is most severe, given the high prevalence of DFO (i.e., up to 60%) in these situations. Both IDSA and IWGDF do not recommend routine bone biopsy in every patient with suspected DFO. However, if the clinical situation remains equivocal or the first-line empiric antibiotic treatment fails, bone biopsy and culture may be helpful. Between microbiology and histopathology for DFO, a recent cross-sectional study showed that histology provided a more accurate diagnosis than microbiology, especially in patients with chronic DFO [50].
Data sources
- Small foot ulcers can lead to infection around the wound, a form of DFI, and many of these cases end up complicating DFO. Differential diagnosis and definite diagnosis of DFO are important for successful treatment. Clinical assessment includes the PTB test or careful wound examination. Plain radiographs are simple but powerful tools for follow-up. Nuclear medicine images such as WBC scintigraphy, 3-phase bone scan, and FDG-PET/CT are used for DFO diagnosis. On the other hand, MRI is still a gold standard diagnosis but not a vital modality for diagnosing DFO. A comprehensive understanding of each tool and proper choice is required among these multiple nuclear medicine modalities. A bone biopsy and culture provide histopathologic and microbiologic findings. Both IDSA and IWGDF do not recommend routine bone biopsy in every patient with suspected DFO. However, if the clinical situation remains equivocal or the first-line empiric antibiotic treatment fails, bone biopsy and culture may be helpful. Based on these numerous diagnosis modalities and indications, the proper choice of diagnostic tool can have favorable treatment outcomes.
Conclusion
-
Conflicts of interest
Chul Hyun Park has been an editorial board member of Journal of Yeungnam Medical Science since 2020. He was not involved in the review process of this manuscript. There is no other conflicts of interest to declare.
-
Funding
This research was supported by a grant of the MD-PhD/Medical Scientist Training Program through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea.
-
Author contributions
Conceptualization, Data curation: SJC, CHP; Investigation: SJC; Formal analysis, Supervision: CHP; Funding acquisition: IW; Methodology: IW, SJC; Writing-original draft: IW; Writing-review & editing: IW.
Notes
- 1. Carracher AM, Marathe PH, Close KL. International diabetes federation 2017. J Diabetes 2018;10:353–6.ArticlePubMedPDF
- 2. Woo I, Park J, Seok H, Kim TG, Moon JS, Chung SM, et al. The fate of antibiotic impregnated cement space in treatment for forefoot osteomyelitis. J Clin Med 2022;11:1976.ArticlePubMedPMC
- 3. Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care 2017;40:136–54.ArticlePubMedPMCPDF
- 4. Lavery LA, Peters EJ, Armstrong DG, Wendel CS, Murdoch DP, Lipsky BA. Risk factors for developing osteomyelitis in patients with diabetic foot wounds. Diabetes Res Clin Pract 2009;83:347–52.ArticlePubMed
- 5. Sohrabi K, Belczyk R. Surgical treatment of diabetic foot and ankle osteomyelitis. Clin Podiatr Med Surg 2022;39:307–19.ArticlePubMed
- 6. Nteleki B, Njokweni M. Want to avoid DFUs? A multidisciplinary team approach works best. J Wound Care 2015;24(5 Suppl 2):8–14.ArticlePubMed
- 7. Berendt AR, Peters EJ, Bakker K, Embil JM, Eneroth M, Hinchliffe RJ, et al. Diabetic foot osteomyelitis: a progress report on diagnosis and a systematic review of treatment. Diabetes Metab Res Rev 2008;24(Suppl 1):S145–61.ArticlePubMed
- 8. Lipsky BA, Aragón-Sánchez J, Diggle M, Embil J, Kono S, Lavery L, et al. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev 2016;32(Suppl 1):45–74.ArticlePubMedPDF
- 9. Lipsky BA, Berendt AR, Deery HG, Embil JM, Joseph WS, Karchmer AW, et al. Diagnosis and treatment of diabetic foot infections. Plast Reconstr Surg 2006;117(7 Suppl):212S–238S.ArticlePubMed
- 10. Lipsky BA, Senneville É, Abbas ZG, Aragón-Sánchez J, Diggle M, Embil JM, et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev 2020;36(Suppl 1):e3280.ArticlePubMedPDF
- 11. Schaper NC, van Netten JJ, Apelqvist J, Bus SA, Fitridge R, Game F, et al. Practical guidelines on the prevention and management of diabetes-related foot disease (IWGDF 2023 update). Diabetes Metab Res Rev 2023 May 27 [Epub]. https://doi.org/10.1002/dmrr.3657.Article
- 12. Boulton AJ, Armstrong DG, Hardman MJ, Malone M, Embil JM, Attinger CE, et al. Diagnosis and management of diabetic foot infections. Arlington (VA): American Diabetes Association; 2020.
- 13. Aragón-Sánchez J, Lipsky BA. Modern management of diabetic foot osteomyelitis: the when, how and why of conservative approaches. Expert Rev Anti Infect Ther 2018;16:35–50.ArticlePubMed
- 14. Giurato L, Meloni M, Izzo V, Uccioli L. Osteomyelitis in diabetic foot: a comprehensive overview. World J Diabetes 2017;8:135–42.ArticlePubMedPMC
- 15. Senneville EM, Lipsky BA, van Asten SA, Peters EJ. Diagnosing diabetic foot osteomyelitis. Diabetes Metab Res Rev 2020;36(Suppl 1):e3250.ArticlePubMedPDF
- 16. Edelson GW, Armstrong DG, Lavery LA, Caicco G. The acutely infected diabetic foot is not adequately evaluated in an inpatient setting. Arch Intern Med 1996;156:2373–8.ArticlePubMed
- 17. Grayson ML, Gibbons GW, Balogh K, Levin E, Karchmer AW. Probing to bone in infected pedal ulcers. A clinical sign of underlying osteomyelitis in diabetic patients. JAMA 1995;273:721–3.ArticlePubMed
- 18. Markanday A. Diagnosing diabetic foot osteomyelitis: narrative review and a suggested 2-step score-based diagnostic pathway for clinicians. Open Forum Infect Dis 2014;1:ofu060.ArticlePubMedPMCPDF
- 19. Lavery LA, Armstrong DG, Peters EJ, Lipsky BA. Probe-to-bone test for diagnosing diabetic foot osteomyelitis: reliable or relic? Diabetes Care 2007;30:270–4.ArticlePubMed
- 20. Lam K, van Asten SA, Nguyen T, La Fontaine J, Lavery LA. Diagnostic accuracy of probe to bone to detect osteomyelitis in the diabetic foot: a systematic review. Clin Infect Dis 2016;63:944–8.ArticlePubMed
- 21. Michail M, Jude E, Liaskos C, Karamagiolis S, Makrilakis K, Dimitroulis D, et al. The performance of serum inflammatory markers for the diagnosis and follow-up of patients with osteomyelitis. Int J Low Extrem Wounds 2013;12:94–9.ArticlePubMedPDF
- 22. Butalia S, Palda VA, Sargeant RJ, Detsky AS, Mourad O. Does this patient with diabetes have osteomyelitis of the lower extremity? JAMA 2008;299:806–13.ArticlePubMed
- 23. Vangaveti VN, Heyes O, Jhamb S, Haleagrahara N, Malabu UH. Usefulness of procalcitonin in diagnosing diabetic foot osteomyelitis: a pilot study. Wounds 2021;33:192–6.ArticlePubMed
- 24. Caruso P, Maiorino MI, Scappaticcio L, Porcellini C, Matrone R, Cirillo P, et al. Biochemical predictors of diabetic foot osteomyelitis: a potential diagnostic role for parathormone. Diabetes Metab Res Rev 2023;39:e3590.ArticlePubMedPDF
- 25. Dinh T, Snyder G, Veves A. Current techniques to detect foot infection in the diabetic patient. Int J Low Extrem Wounds 2010;9:24–30.ArticlePubMed
- 26. Alazraki N, Dalinka MK, Berquist TH, Daffner RJ, De Smet AA, el-Khoury GY, et al. Imaging diagnosis of osteomyelitis in patients with diabetes mellitus. American College of Radiology. ACR Appropriateness Criteria. Radiology 2000;215(Suppl):303–10.
- 27. Tan PL, Teh J. MRI of the diabetic foot: differentiation of infection from neuropathic change. Br J Radiol 2007;80:939–48.ArticlePubMed
- 28. Aragón-Sánchez J, Lipsky BA, Lázaro-Martínez JL. Diagnosing diabetic foot osteomyelitis: is the combination of probe-to-bone test and plain radiography sufficient for high-risk inpatients? Diabet Med 2011;28:191–4.ArticlePubMed
- 29. Donovan A, Schweitzer ME. Use of MR imaging in diagnosing diabetes-related pedal osteomyelitis. Radiographics 2010;30:723–36.ArticlePubMed
- 30. Lauri C, Leone A, Cavallini M, Signore A, Giurato L, Uccioli L. Diabetic foot infections: the diagnostic challenges. J Clin Med 2020;9:1779.ArticlePubMedPMC
- 31. Wukich DK, Schaper NC, Gooday C, Bal A, Bem R, Chhabra A, et al. Guidelines on the diagnosis and treatment of active Charcot neuro-osteoarthropathy in persons with diabetes mellitus (IWGDF 2023). Diabetes Metab Res Rev 2023 May 23 [Epub]. https://doi.org/10.1002/dmrr.3646.Article
- 32. La Fontaine J, Bhavan K, Jupiter D, Lavery LA, Chhabra A. Magnetic resonance imaging of diabetic foot osteomyelitis: imaging accuracy in biopsy-proven disease. J Foot Ankle Surg 2021;60:17–20.ArticlePubMed
- 33. Ertugrul BM, Lipsky BA, Savk O. Osteomyelitis or Charcot neuro-osteoarthropathy?: differentiating these disorders in diabetic patients with a foot problem. Diabet Foot Ankle 2013;4:21855.ArticlePubMedPMC
- 34. Lázaro-Martínez JL, Tardáguila-García A, García-Klepzig JL. Diagnostic and therapeutic update on diabetic foot osteomyelitis. Endocrinol Diabetes Nutr 2017;64:100–8.ArticlePubMed
- 35. Daneshvar K, Anwander H. Diagnostic imaging of diabetic foot disorders. Foot Ankle Clin 2022;27:513–27.ArticlePubMed
- 36. Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012;54:e132–73.ArticlePubMed
- 37. Sella EJ, Grosser DM. Imaging modalities of the diabetic foot. Clin Podiatr Med Surg 2003;20:729–40.ArticlePubMed
- 38. Nawaz A, Torigian DA, Siegelman ES, Basu S, Chryssikos T, Alavi A. Diagnostic performance of FDG-PET, MRI, and plain film radiography (PFR) for the diagnosis of osteomyelitis in the diabetic foot. Mol Imaging Biol 2010;12:335–42.ArticlePubMedPDF
- 39. Maurer AH, Millmond SH, Knight LC, Mesgarzadeh M, Siegel JA, Shuman CR, et al. Infection in diabetic osteoarthropathy: use of indium-labeled leukocytes for diagnosis. Radiology 1986;161:221–5.ArticlePubMed
- 40. Keenan AM, Tindel NL, Alavi A. Diagnosis of pedal osteomyelitis in diabetic patients using current scintigraphic techniques. Arch Intern Med 1989;149:2262–6.ArticlePubMed
- 41. Newman LG, Waller J, Palestro CJ, Hermann G, Klein MJ, Schwartz M, et al. Leukocyte scanning with 111In is superior to magnetic resonance imaging in diagnosis of clinically unsuspected osteomyelitis in diabetic foot ulcers. Diabetes Care 1992;15:1527–30.ArticlePubMedPDF
- 42. Unal SN, Birinci H, Baktiroğlu S, Cantez S. Comparison of Tc-99m methylene diphosphonate, Tc-99m human immune globulin, and Tc-99m-labeled white blood cell scintigraphy in the diabetic foot. Clin Nucl Med 2001;26:1016–21.ArticlePubMed
- 43. Lauri C, Glaudemans AW, Campagna G, Keidar Z, Muchnik Kurash M, Georga S, et al. Comparison of white blood cell scintigraphy, FDG PET/CT and MRI in suspected diabetic foot infection: results of a large retrospective multicenter study. J Clin Med 2020;9:1645.ArticlePubMedPMC
- 44. Termaat MF, Raijmakers PG, Scholten HJ, Bakker FC, Patka P, Haarman HJ. The accuracy of diagnostic imaging for the assessment of chronic osteomyelitis: a systematic review and meta-analysis. J Bone Joint Surg Am 2005;87:2464–71.Article
- 45. Treglia G, Sadeghi R, Annunziata S, Caldarella C, Bertagna F, Giovanella L. Diagnostic performance of fluorine-18-fluorodeoxyglucose positron emission tomography in the postchemotherapy management of patients with seminoma: systematic review and meta-analysis. Biomed Res Int 2014;2014:852681.ArticlePubMedPMCPDF
- 46. Senneville E, Gaworowska D, Topolinski H, Devemy F, Nguyen S, Singer B, et al. Outcome of patients with diabetes with negative percutaneous bone biopsy performed for suspicion of osteomyelitis of the foot. Diabet Med 2012;29:56–61.ArticlePubMed
- 47. Cecilia-Matilla A, Lázaro-Martínez JL, Aragón-Sánchez J, García-Morales E, García-Álvarez Y, Beneit-Montesinos JV. Histopathologic characteristics of bone infection complicating foot ulcers in diabetic patients. J Am Podiatr Med Assoc 2013;103:24–31.ArticlePubMedPDF
- 48. Elamurugan TP, Jagdish S, Kate V, Chandra Parija S. Role of bone biopsy specimen culture in the management of diabetic foot osteomyelitis. Int J Surg 2011;9:214–6.ArticlePubMed
- 49. Couturier A, Chabaud A, Desbiez F, Descamps S, Petrosyan E, Letertre-Gilbert P, et al. Comparison of microbiological results obtained from per-wound bone biopsies versus transcutaneous bone biopsies in diabetic foot osteomyelitis: a prospective cohort study. Eur J Clin Microbiol Infect Dis 2019;38:1287–91.ArticlePubMedPDF
- 50. Tardáguila-García A, Sanz-Corbalán I, García-Morales E, García-Álvarez Y, Molines-Barroso RJ, Lázaro-Martínez JL. Diagnostic accuracy of bone culture versus biopsy in diabetic foot osteomyelitis. Adv Skin Wound Care 2021;34:204–8.ArticlePubMed
References
Figure & Data
References
Citations

- Unveiling the challenges of diabetic foot infections: diagnosis, pathogenesis, treatment, and rehabilitation
Chul Hyun Park
Journal of Yeungnam Medical Science.2023; 40(4): 319. CrossRef
- Figure
- Related articles
-
- The pathophysiology of diabetic foot: a narrative review
- Management and rehabilitation of moderate-to-severe diabetic foot infection: a narrative review
- Management of diabetic foot ulcers: a narrative review
- Anatomical endoscopic enucleation of the prostate for bladder outlet obstruction: a narrative review
- Current diagnosis and treatment of vestibular neuritis: a narrative review

 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine PubReader
PubReader ePub Link
ePub Link Cite
Cite