Indexed in: ESCI, Scopus, PubMed,
PubMed Central, CAS, DOAJ, KCI
PubMed Central, CAS, DOAJ, KCI
FREE article processing charge

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Volume 41(1); 2024 > Article
-
Review article
Breakthrough pain and rapid-onset opioids in patients with cancer pain: a narrative review -
Jinseok Yeo

-
Journal of Yeungnam Medical Science 2024;41(1):22-29.
DOI: https://doi.org/10.12701/jyms.2023.00367
Published online: June 30, 2023
Department of Anesthesiology and Pain Medicine, Kyungpook National University Chilgok Hospital, School of Medicine, Kyungpook National University, Daegu, Korea
- Corresponding author: Jinseok Yeo, MD Department of Anesthesiology and Pain Medicine, Kyungpook National University Chilgok Hospital, School of Medicine, Kyungpook National University, 807 Hoguk-ro, Buk-gu, Daegu 41404, Korea Tel : +82-53-200-2644 • Fax: +82-53-200-2027 • E-mail: painfree@gmail.com
• Received: April 3, 2023 • Revised: May 11, 2023 • Accepted: May 19, 2023
Copyright © 2024 Yeungnam University College of Medicine, Yeungnam University Institute of Medical Science
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 5,448 Views
- 230 Download
Abstract
- Breakthrough pain is transitory pain that occurs despite the use of opioids for background pain control. Breakthrough pain occurs in 40% to 80% of patients with cancer pain. Despite effective analgesic therapy, patients and their caregivers often feel that their pain is not sufficiently controlled. Therefore, an improved understanding of breakthrough pain and its management is essential for all physicians caring for patients with cancer. This article reviews the definition, clinical manifestations, accurate diagnostic strategies, and optimal treatment options for breakthrough pain in patients with cancer. This review focuses on the efficacy and safety of rapid-onset opioids, which are the primary rescue drugs for breakthrough pain.
- Pain is a common symptom in patients with cancer. Cancer pain can occur during anticancer treatment, after curative treatment, or in advanced, metastatic, or terminal disease [1]. Cancer pain occurs in 24% to 60% of patients receiving active treatment, and the incidence of pain is 58% to 69% in advanced stages. This rate has not decreased in decades [2].
- Cancer pain is a complex phenomenon resulting from several factors, including genetic variants, microenvironmental alterations, nociceptor activation, tumor growth, and tumor metastasis [3]. It can be classified according to the pathophysiology (nociceptive and neuropathic), cause (related or unrelated to the disease and its treatment), temporal characteristics, and nature of the pain experience [3].
- Pain control in patients with cancer is essential but remains challenging for clinicians [4]. Cancer pain management has improved over the decades; however, one-third of patients with cancer do not receive pain medication because of barriers to reporting pain, lack of assessment, and undermanagement [5]. Breakthrough pain is commonly described as transitory pain that occurs despite adequate background pain control with opioids [6]. It commonly occurs in patients with cancer who suffer from pain, leading to complications and reduced quality of life. Despite effective analgesic therapy, patients and their caregivers often feel that the pain is not adequately controlled. In addition, breakthrough pain increases healthcare utilization and costs [7]. Therefore, an improved understanding of breakthrough pain and its management is essential for all physicians caring for patients with cancer. This review aims to provide a comprehensive overview of the definition, clinical presentation, appropriate diagnosis, and treatment strategies for breakthrough pain in patients with cancer, focusing on the efficacy and safety of rapid-onset opioids.
Introduction
- Breakthrough pain was first defined in 1990 as “a transient increase in pain to greater than moderate intensity, which occurred on a baseline pain of moderate intensity or less” [8]. This definition excludes patients with severe baseline pain, indicating uncontrolled pain. Some researchers argue that breakthrough pain should be defined as pain that occurs despite regular opioid treatment for baseline pain, because breakthrough pain indicates a transient increase in pain breaking through the background pain protected by opioids. Based on this opinion, breakthrough pain is defined as “a transitory flare of pain superimposed on an otherwise stable pain pattern in patients treated with opioids” [9]. There is no consensus on the definition of breakthrough pain. The most widely accepted definition of breakthrough pain is that of the Association for Palliative Medicine of Great Britain and Ireland (APM), namely “a transient exacerbation of pain that occurs spontaneously or in relation to a specific predictable or unpredictable trigger despite relatively stable and adequately controlled background pain” [6,10]. According to this APM definition, background pain should be assessed and appropriately controlled before breakthrough pain is diagnosed.
- The National Comprehensive Cancer Network (NCCN) guideline defines breakthrough pain as “pain that fails to be controlled or breaks through a regimen of regularly scheduled analgesics” [11]. Pain at the end of a regular opioid dose interval is known as end-of-dose failure pain. End-of-dose failure pain is frequently related to an underdose of the opioids used to control background pain and occurs more often during the titration phase [6]. The NCCN guideline includes end-of-dose failure pain as a subtype of breakthrough pain, but the APM definition excludes end-of-dose failure pain from breakthrough pain [6,11].
- In 2002, an Expert Working Group of the European Association for Palliative Care suggested that the term “breakthrough pain” should be replaced by “episodic pain” [12]. Patients with cancer may experience a transient exacerbation of pain in the absence of background pain. Therefore, the experts suggested that episodic pain should be defined as any transient exacerbation of pain in patients with cancer [13].
Definition of breakthrough pain
- Breakthrough pain is a spectrum of heterogeneous conditions that vary among and within individuals according to different clinical features, disease stages, and treatments [14]. Tumor growth, cancer treatment, metastasis, and comorbidities can cause breakthrough pain, which can be nociceptive, neuropathic, or mixed. It negatively affects daily living in 80% of the patients with cancer pain [15]. Two to three episodes of breakthrough pain per day have been reported. It is most prevalent in the late morning, with 60% of pain episodes occurring during the daytime [16]. The proposed mechanisms for the circadian rhythm of breakthrough pain flare-ups are as follows: (1) late morning is the time of maximum physical activity, (2) pain sensitivity has a circadian rhythm, and (3) the pharmacokinetics or pharmacodynamics of opioids have a circadian rhythm [16]. The pain usually peaks within 5 minutes and is moderate-to-severe [14,17]. Peak pain intensity rarely occurs after 15 minutes [18,19]. Untreated breakthrough pain lasts for a median of 60 minutes and a maximum of 180 minutes [14].
- The overall prevalence of breakthrough pain was reported to be 59.2% [20]. It was lower in outpatients (39.9%) and higher in hospice patients (80.5%). This difference was mainly due to disease progression. Additionally, hospice clinicians have more knowledge and experience in identifying breakthrough pain, which increases the likelihood of reporting it. The prevalence of breakthrough pain has decreased in recent publications compared to that in previous publications [21]. This decrease may be due to improvements in diagnostic criteria, especially the exclusion of end-of-dose failure, control of background pain, and use of analgesics.
- Breakthrough pain is categorized as incident or spontaneous. Incident pain is predictable. It can be triggered by voluntary movements, such as walking; involuntary movements, such as coughing; or therapeutic interventions, such as wound dressing. Spontaneous pain is unpredictable because it has no identifiable cause. Davies et al. reported that 44% of breakthrough pain cases were incident, 41.5% were spontaneous, and 14.5% were mixed [14]. Episodes of breakthrough pain did not differ between the two types. However, incident pain has a shorter duration and faster onset, whereas spontaneous pain has a gradual onset and longer duration [21]. The characteristics of the two types of pain are summarized in Table 1.
Clinical presentation of breakthrough pain
- Adequate patient assessment is essential to determine the cause, severity, and characteristics of pain [6]. However, distinguishing breakthrough pain from poorly controlled background pain remains challenging [22]. Breakthrough pain is diagnosed based on multiple sources. A patient’s history is an essential component of the diagnosis of breakthrough pain. In patients with normal cognitive function, self-reporting is the best source of information regarding breakthrough pain. A pain diary is valuable for the assessment and monitoring of breakthrough pain [17]. It provides the date and time of each episode, duration and intensity of pain, rescue dose administration, pain relief, and side effects. However, patient adherence to keeping a diary is generally poor. The Numerical Rating Scale (NRS) is a useful tool for measuring pain intensity. The NRS is associated with higher adherence and better responsiveness than the visual analog and verbal rating scales [23]. Patients with cancer preferred to use the NRS to measure pain exacerbations, and the NRS performed better in distinguishing between background pain and peak pain intensity [24]. Patients usually request breakthrough pain medications when their NRS pain scores are >7 [25]. The Brief Pain Inventory and McGill Pain Questionnaire are multidimensional tools that can provide information on the location of pain, daily function, and treatment effects. These tools are complex and do not differentiate breakthrough pain from background pain, although they provide helpful information [26].
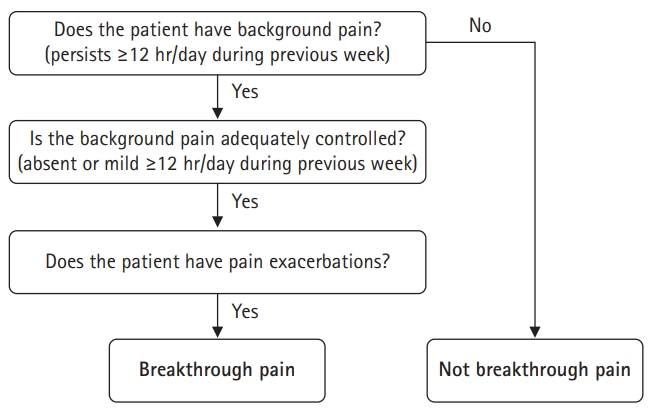
- A diagnostic algorithm is valuable for screening breakthrough pain (Fig. 1) [6]. It has a high positive predictive value (0.84) when using mild as a cutoff level to define controlled background pain [27]. However, the positive predictive value was lower when using moderate as the cutoff level [27].
- The Alberta Breakthrough Pain Assessment Tool (ABPAT) and Breakthrough Pain Assessment Tool (BAT) are specific tools for assessing breakthrough pain. The ABPAT consists of 15 self-answer questions on breakthrough pain, asking about the association with background pain, previous pain experience time, pain frequency, peak pain intensity, pain location, pain quality, cause of pain, and pain predictability [28]. The BAT comprises 14 questions evaluating pain and current pain treatments for previously diagnosed breakthrough pain [29]. ABPAT is usually used for research, and BAT is used to improve pain management in clinical settings [26].
Assessment of breakthrough pain
- Because of the heterogeneous nature of breakthrough pain, its management should be individualized [6]. Management should be considered based on the cause, pathophysiology, and clinical features of the pain. A direct effect of cancer is the most common cause of cancer-related pain. Treatment of underlying causes, such as bone metastasis, includes conventional radiotherapy, bisphosphonates, and nuclear factor kappa-B ligand-receptor activator inhibition [30]. Avoiding the cause of pain reduces incident pain when using orthotic devices.
- Modification of the background analgesic regimen, including titration of opioid analgesics, switching of opioid analgesics, and addition of adjuvant analgesics such as antiepileptics for neuropathic pain or antispasmodics for visceral pain, also reduces breakthrough pain [6]. The patient’s condition, including disease stage, physical performance, and personal preferences, should be considered to manage breakthrough pain effectively [6]. Younger patients, those with high physical performance, and those without advanced disease were not satisfied with breakthrough pain management in a previous study and experienced more interference in daily living activities due to breakthrough pain [31].
- Non-pharmacological methods include massage, heat or cold application, relaxation or distraction techniques, mindfulness intervention, and physical therapy [25]. However, there is limited evidence supporting the use of these methods [6].
- Interventions such as neural blockade, chemical neurolysis, neuraxial drug infusion, direct tumor ablation, cementoplasty, and surgery may help manage breakthrough pain [6].
- Opioids are the mainstay of analgesics used for cancer pain treatment. The use of opioids as rescue medications is the cornerstone of controlling breakthrough pain episodes [6]. Orally administered morphine and short-acting opioids are the traditional backbones for the pharmacological management of breakthrough pain. Orally administered morphine or oxycodone has a slow onset of action (onset of analgesia, 30–45 minutes) and a prolonged duration of effect (3–6 hours) [32]. These characteristics of oral opioids can delay the management of breakthrough pain and increase the incidence of adverse effects.
- Consequently, alternative methods may be better for treating this type of pain [33]. Intravenous or subcutaneous administration of opioids has been attempted to manage breakthrough pain and satisfy hospitalized patients [34]. Intravenous or subcutaneous administration of opioids can provide rapid analgesia for breakthrough pain. Infusion using patient-controlled analgesia devices has also been attempted; however, these methods are limited to primary care settings.
- The demand for more rapid and accessible breakthrough pain relief has led to the development of rapid-onset opioid therapies. Transmucosal routes deliver drugs more rapidly than oral routes in a noninvasive manner. The oral and nasal mucosa are easily accessible and more permeable than the skin. They are rich in blood supply, enabling the fast absorption of drugs, and the absorbed drugs have the advantage of bypassing first-pass metabolism. Fentanyl, a highly lipophilic µ receptor agonist with high potency, quickly crosses the blood–brain barrier to provide fast analgesia [35]. It has a rapid blood–brain equilibration time constant of 5 to 6 minutes [36]. Transmucosal administration of fentanyl provides an immediate analgesic effect that closely mimics the duration of breakthrough pain episodes and increases bioavailability by bypassing first-pass metabolism. The following are commonly utilized forms of transmucosal administered fentanyl: oral transmucosal fentanyl citrate (OTFC), fentanyl buccal tablet (FBT), sublingual fentanyl (SLF), fentanyl intranasal spray (INFS), and fentanyl pectin nasal spray (FPNS). The characteristics of the rapid-onset opioids are shown in Table 2.
- OTFC is a sweetened fentanyl citrate lozenge on a stick to help the patient spread the medication over the buccal mucosa. The buccal mucosa only absorbs 25% of the administered dose. The remaining 75% of the OTFC dose is swallowed and slowly absorbed through the gastrointestinal tract. Approximately two-thirds of the absorbed dose is eliminated through first-pass metabolism. The bioavailability of OTFC is approximately 50% of the total dose. OTFC must be taken for 15 minutes. Absorption is reduced if the patient has decreased saliva levels, applies the OTFC to the tongue or gums rather than the buccal mucosa, chews the OTFC, ingests liquids that alter the oral pH before OTFC administration, or applies the product for less than or longer than 15 minutes [18]. The time to maximum plasma concentration (Tmax) is 20 to 40 minutes after administration, depending on the dose [37]. In cases of incomplete pain relief, a second dose may be administered 15 minutes after complete dissolution of the first lozenge. The sugar in the lozenges increases the risk of dental decay.
- FBT is an effervescent tablet intended to modify the pH of the buccal cavity and enhance drug absorption. This alteration enhances the dissolution of ionized fentanyl and absorption of non-ionized fentanyl across the buccal mucosa [38]. FBT is inserted between the upper cheek and gum within the buccal cavity above the rear molars. FBT dissolves in the buccal mucosa for 14 to 25 minutes; 48% of the administered dose is absorbed by the buccal mucosa, whereas 52% is absorbed by the gastrointestinal tract [39]. The absolute bioavailability is 65%. Pain intensity is reduced as early as 10 minutes after administration [40].
- SLF is a tablet that contains fentanyl citrate mixed with carrier particles and a mucoadhesive agent. It uses a rapid disintegration system and dissolves under the tongue within 2 minutes. The drug is rapidly absorbed through the oral and sublingual mucosa, obtaining a detectable plasma concentration within 10 minutes [40]. The estimated bioavailability of SLF is 70% because of its rapid absorption through the sublingual mucosa [40].
- Fentanyl administration through the oral mucosa requires time to dissolve and adequate amounts of saliva. However, salivary gland dysfunction and xerostomia are common in patients with cancer, and fentanyl absorption across the oral mucosa may pose a challenge [41]. The nasal mucosa has a large surface area and an extensive blood supply, facilitating the absorption of lipophilic drugs. The nasal route bypasses first-pass metabolism and delivers opioids directly to the site of action in the central nervous system through the olfactory and trigeminal nerves, vessels, cerebrospinal fluid, and lymphatic fluid.
- INFS has a pH of 6.4 to minimize nasal mucosa irritation and is administered at 100 μL per nostril separately to prevent pharynx runoff. Moreover, 50, 100, or 200 μg of fentanyl is delivered in 100 μL per spray. Its bioavailability is 89% because less of the administered dose is swallowed than with transbuccal or sublingual formulations [42]. INFS has a fast onset of action (5–10 minutes), a Tmax of 12 to 15 minutes, and a duration of action of approximately 2 hours. The long-term use of INFS can cause nasal congestion, epistaxis, and changes in nasociliary function [43].
- FPNS is a nasal spray containing pectin as a food additive. It reduces local irritation and improves the nasal mucosal penetration of fentanyl. The pectin-containing fentanyl citrate solution applied to the nasal mucosa transforms into a gel that prolongs the residence time at the application site and prevents intranasal runoff. The bioavailability of FPNS is 70% [44]. It attenuates the peak plasma concentration of fentanyl and has a prolonged elimination half-life compared to that of INFS. FPNS has a rapid Tmax (15–21 minutes) and long elimination half-life (15–25 hours). The recommended starting dose of FPNS is 100 μg. A 2-hour interval must be observed before treating any subsequent episode of breakthrough pain because of the long elimination half-life of FPNS.
- All types of transmucosal fentanyl decrease breakthrough pain within 30 minutes. OTFC, FBT, INFS, and FPNS significantly reduce pain within 15 minutes. SLF shows significant pain reduction at 30 minutes, but not at 15 minutes. INFS shows superior efficacy compared to all other medications at 15 and 30 minutes. INFS also shows greater efficacy at 5 minutes than FBT and OTFC but not FPNS [45]. INFS and FPNS provide the fastest meaningful pain relief even though they are administered at relatively low doses, possibly because of their faster analgesic effects [46]. Nasal transmucosal fentanyl is preferred in patients with severe mucositis [46].
- Most patients were satisfied with transmucosal fentanyl administered for breakthrough pain. Patients were more likely to use FPNS and SLF than OTFC [46]. A study indicated that breakthrough pain management with rapid onset transmucosal fentanyl improved quality of life, including physical and emotional status [47].
- For patients who are opioid tolerant, transmucosal fentanyl is recommended for the treatment of incident pain not relieved by conventional immediate-release opioids, but it is not recommended for use when background pain control is inadequate [11]. Starting with the lowest possible dose of transmucosal fentanyl and up-titrating is recommended. However, repeated dosing during titration may prolong the duration of uncontrolled pain. This discourages patients who may refuse treatment and increases the uncertainty, inconvenience, and cost of care. A recent study showed that the opioid doses required for breakthrough pain were significantly associated with those required for background pain [48]. The treatment of breakthrough pain was attempted by starting the first dose of transmucosal fentanyl based on the total daily dose of opioids. This method appears to be effective and well-tolerated based on available evidence [48,49].
- It is recommended that no more than four doses of all forms of transmucosal fentanyl be administered per day. However, there is no pharmacological reason for this limit, and clinicians frequently administer more than four doses daily to appropriate patients [10]. However, a higher frequency of breakthrough pain episodes was associated with suboptimal pain management [50]. Therefore, optimization of background analgesia may reduce the frequency of breakthrough pain.
- Fentanyl is highly addictive, carries a risk of abuse, and increases mortality in patients who are not opioid tolerant [51]. Therefore, transmucosal fentanyl is recommended for patients who are opioid tolerant and take >60 mg of oral morphine or equivalent daily. Recent studies have demonstrated the safety and efficacy of transmucosal fentanyl for managing breakthrough pain in patients receiving low-dose opioids [52]. Opioid-use disorder in patients with cancer is low (8%), and evidence that transmucosal fentanyl is more addictive is scarce [53]. Despite concerns regarding opioid abuse and dependence, clinicians should not hesitate to use opioids to manage cancer pain in patients with only a few months to live.
Management of breakthrough pain and rapid-onset opioids
- The management of breakthrough pain in cancer remains challenging. It requires individualized treatment based on the cause, pathophysiology, and clinical features of the pain. Strategies for managing breakthrough pain include the use of oral opioids, adjuvant analgesics, neuraxial opioids, orthotic devices, interventions, and surgery. The primary treatment is a rescue medication using rapid-onset opioids with adequate background pain control. Clinicians should use their knowledge of rapid-onset opioids to identify the formulation that best treats a patient with breakthrough pain.
Conclusion
-
Conflicts of interest
There are no potential conflict of interest relevant to this article.
-
Funding
None.
Article information
Table 1.Characteristics of breakthrough pain
Table 2.Characteristics of rapid onset opioids
- 1. van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage 2016;51:1070–90.ArticlePubMed
- 2. van den Beuken-van Everdingen MH, van Kuijk SM, Janssen DJ, Joosten EA. Treatment of pain in cancer: towards personalised medicine. Cancers (Basel) 2018;10:502.ArticlePubMedPMC
- 3. Müller-Schwefe G, Ahlbeck K, Aldington D, Alon E, Coaccioli S, Coluzzi F, et al. Pain in the cancer patient: different pain characteristics CHANGE pharmacological treatment requirements. Curr Med Res Opin 2014;30:1895–908.ArticlePubMed
- 4. Caraceni A, Martini C, Zecca E, Portenoy RK, Ashby MA, Hawson G, et al. Breakthrough pain characteristics and syndromes in patients with cancer pain: an international survey. Palliat Med 2004;18:177–83.ArticlePubMedPDF
- 5. Greco MT, Roberto A, Corli O, Deandrea S, Bandieri E, Cavuto S, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol 2014;32:4149–54.ArticlePubMed
- 6. Davies AN, Dickman A, Reid C, Stevens AM, Zeppetella G; Science Committee of the Association for Palliative Medicine of Great Britain and Ireland. The management of cancer-related breakthrough pain: recommendations of a task group of the Science Committee of the Association for Palliative Medicine of Great Britain and Ireland. Eur J Pain 2009;13:331–8.ArticlePubMed
- 7. Fortner BV, Demarco G, Irving G, Ashley J, Keppler G, Chavez J, et al. Description and predictors of direct and indirect costs of pain reported by cancer patients. J Pain Symptom Manage 2003;25:9–18.ArticlePubMed
- 8. Portenoy RK, Hagen NA. Breakthrough pain: definition, prevalence and characteristics. Pain 1990;41:273–81.ArticlePubMed
- 9. Coluzzi PH. Cancer pain management: newer perspectives on opioids and episodic pain. Am J Hosp Palliat Care 1998;15:13–22.ArticlePubMedPDF
- 10. Davies AN, Elsner F, Filbet MJ, Porta-Sales J, Ripamonti C, Santini D, et al. Breakthrough cancer pain (BTcP) management: a review of international and national guidelines. BMJ Support Palliat Care 2018;8:241–9.ArticlePubMed
- 11. National Comprehensive Cancer Network (NCCN). Adult cancer pain: version 1 [Internet]. Fort Washington, PA: NCCN; 2023 [cited 2023 Apr 23]. https://www.nccn.org/professionals/physician_gls/pdf/pain.pdf.
- 12. Mercadante S, Radbruch L, Caraceni A, Cherny N, Kaasa S, Nauck F, et al. Episodic (breakthrough) pain: consensus conference of an expert working group of the European Association for Palliative Care. Cancer 2002;94:832–9.ArticlePubMed
- 13. Løhre ET, Klepstad P, Bennett MI, Brunelli C, Caraceni A, Fainsinger RL, et al. From “breakthrough” to “episodic” cancer pain?: a European Association for Palliative Care Research Network Expert Delphi Survey toward a common terminology and classification of transient cancer pain exacerbations. J Pain Symptom Manage 2016;51:1013–9.ArticlePubMed
- 14. Davies A, Buchanan A, Zeppetella G, Porta-Sales J, Likar R, Weismayr W, et al. Breakthrough cancer pain: an observational study of 1000 European oncology patients. J Pain Symptom Manage 2013;46:619–28.ArticlePubMed
- 15. Mercadante S, Lazzari M, Reale C, Cuomo A, Fusco F, Marchetti P, et al. Italian Oncological Pain Survey (IOPS): a multicentre Italian study of breakthrough pain performed in different settings. Clin J Pain 2015;31:214–21.ArticlePubMed
- 16. Saini A, Tucci M, Tampellini M, Maina D, Bouraouia K, Giuliano PL, et al. Circadian variation of breakthrough pain in cancer patients. Eur J Pain 2013;17:264–70.ArticlePubMed
- 17. Boceta J, De la Torre A, Samper D, Farto M, Sánchez-de la Rosa R. Consensus and controversies in the definition, assessment, treatment and monitoring of BTcP: results of a Delphi study. Clin Transl Oncol 2016;18:1088–97.ArticlePubMedPMCPDF
- 18. Smith H. A comprehensive review of rapid-onset opioids for breakthrough pain. CNS Drugs 2012;26:509–35.ArticlePubMedPMC
- 19. Davies A, Zeppetella G, Andersen S, Damkier A, Vejlgaard T, Nauck F, et al. Multi-centre European study of breakthrough cancer pain: pain characteristics and patient perceptions of current and potential management strategies. Eur J Pain 2011;15:756–63.ArticlePubMedPDF
- 20. Deandrea S, Corli O, Consonni D, Villani W, Greco MT, Apolone G. Prevalence of breakthrough cancer pain: a systematic review and a pooled analysis of published literature. J Pain Symptom Manage 2014;47:57–76.ArticlePubMed
- 21. Svendsen KB, Andersen S, Arnason S, Arnér S, Breivik H, Heiskanen T, et al. Breakthrough pain in malignant and non-malignant diseases: a review of prevalence, characteristics and mechanisms. Eur J Pain 2005;9:195–206.ArticlePubMed
- 22. Soden K, Ali S, Alloway L, Barclay D, Perkins P, Barker S. How do nurses assess and manage breakthrough pain in specialist palliative care inpatient units?: a multicentre study. Palliat Med 2010;24:294–8.ArticlePubMedPDF
- 23. Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, et al. Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage 2011;41:1073–93.ArticlePubMed
- 24. Brunelli C, Zecca E, Martini C, Campa T, Fagnoni E, Bagnasco M, et al. Comparison of numerical and verbal rating scales to measure pain exacerbations in patients with chronic cancer pain. Health Qual Life Outcomes 2010;8:42.ArticlePubMedPMC
- 25. Mercadante S, Adile C, Torta R, Varetto A, Fulfaro F, Giarratano A, et al. Meaningful cut-off pain intensity for breakthrough pain changes in advanced cancer patients. Curr Med Res Opin 2013;29:93–7.ArticlePubMed
- 26. Daeninck P, Gagnon B, Gallagher R, Henderson JD, Shir Y, Zimmermann C, et al. Canadian recommendations for the management of breakthrough cancer pain. Curr Oncol 2016;23:96–108.ArticlePubMedPMCPDF
- 27. Webber K, Davies AN, Cowie MR. Accuracy of a diagnostic algorithm to diagnose breakthrough cancer pain as compared with clinical assessment. J Pain Symptom Manage 2015;50:495–500.ArticlePubMed
- 28. Hagen NA, Stiles C, Nekolaichuk C, Biondo P, Carlson LE, Fisher K, et al. The Alberta Breakthrough Pain Assessment Tool for cancer patients: a validation study using a Delphi process and patient think-aloud interviews. J Pain Symptom Manage 2008;35:136–52.ArticlePubMed
- 29. Webber K, Davies AN, Zeppetella G, Cowie MR. Development and validation of the breakthrough pain assessment tool (BAT) in cancer patients. J Pain Symptom Manage 2014;48:619–31.ArticlePubMed
- 30. Ahmad I, Ahmed MM, Ahsraf MF, Naeem A, Tasleem A, Ahmed M, et al. Pain management in metastatic bone disease: a literature review. Cureus 2018;10:e3286.ArticlePubMedPMC
- 31. Mazzotta M, Filetti M, Piras M, Mercadante S, Marchetti P, Giusti R. Patients’ satisfaction with breakthrough cancer pain therapy: a secondary analysis of IOPS-MS Study. Cancer Manag Res 2022;14:1237–45.ArticlePubMedPMCPDF
- 32. Mercadante S. Pharmacotherapy for breakthrough cancer pain. Drugs 2012;72:181–90.ArticlePubMed
- 33. Zeppetella G. Dynamics of breakthrough pain vs. pharmacokinetics of oral morphine: implications for management. Eur J Cancer Care (Engl) 2009;18:331–7.ArticlePubMed
- 34. Mercadante S, Villari P, Ferrera P, Porzio G, Aielli F, Verna L, et al. Safety and effectiveness of intravenous morphine for episodic breakthrough pain in patients receiving transdermal buprenorphine. J Pain Symptom Manage 2006;32:175–9.ArticlePubMed
- 35. Stanley TH. The fentanyl story. J Pain 2014;15:1215–26.ArticlePubMed
- 36. Lötsch J. Pharmacokinetic-pharmacodynamic modeling of opioids. J Pain Symptom Manage 2005;29(5 Suppl):S90–103.ArticlePubMed
- 37. Streisand JB, Busch MA, Egan TD, Smith BG, Gay M, Pace NL. Dose proportionality and pharmacokinetics of oral transmucosal fentanyl citrate. Anesthesiology 1998;88:305–9.ArticlePubMed
- 38. Darwish M, Messina J. Clinical pharmacology of fentanyl buccal tablet for the treatment of breakthrough pain. Expert Rev Clin Pharmacol 2008;1:39–47.ArticlePubMed
- 39. Portenoy RK, Taylor D, Messina J, Tremmel L. A randomized, placebo-controlled study of fentanyl buccal tablet for breakthrough pain in opioid-treated patients with cancer. Clin J Pain 2006;22:805–11.ArticlePubMed
- 40. Chwieduk CM, McKeage K. Fentanyl sublingual: in breakthrough pain in opioid-tolerant adults with cancer. Drugs 2010;70:2281–8.ArticlePubMed
- 41. Davies A, Mundin G, Vriens J, Webber K, Buchanan A, Waghorn M. The influence of low salivary flow rates on the absorption of a sublingual fentanyl citrate formulation for breakthrough cancer pain. J Pain Symptom Manage 2016;51:538–45.ArticlePubMed
- 42. Foster D, Upton R, Christrup L, Popper L. Pharmacokinetics and pharmacodynamics of intranasal versus intravenous fentanyl in patients with pain after oral surgery. Ann Pharmacother 2008;42:1380–7.ArticlePubMedPDF
- 43. Panagiotou I, Mystakidou K. Intranasal fentanyl: from pharmacokinetics and bioavailability to current treatment applications. Expert Rev Anticancer Ther 2010;10:1009–21.ArticlePubMed
- 44. Lötsch J, Walter C, Parnham MJ, Oertel BG, Geisslinger G. Pharmacokinetics of non-intravenous formulations of fentanyl. Clin Pharmacokinet 2013;52:23–36.ArticlePubMedPDF
- 45. Zeppetella G, Davies A, Eijgelshoven I, Jansen JP. A network meta-analysis of the efficacy of opioid analgesics for the management of breakthrough cancer pain episodes. J Pain Symptom Manage 2014;47:772–85.ArticlePubMed
- 46. Mercadante S, Adile C, Masedu F, Marchetti P, Costanzi A, Aielli F. Factors influencing the use of opioids for breakthrough cancer pain: a secondary analysis of the IOPS-MS study. Eur J Pain 2019;23:719–26.ArticlePubMedPDF
- 47. Cuomo A, Cascella M, Forte CA, Bimonte S, Esposito G, De Santis S, et al. Careful breakthrough cancer pain treatment through rapid-onset transmucosal fentanyl improves the quality of life in cancer patients: results from the BEST Multicenter Study. J Clin Med 2020;9:1003.ArticlePubMedPMC
- 48. Mercadante S, Gatti A, Porzio G, Lo Presti C, Aielli F, Adile C, et al. Dosing fentanyl buccal tablet for breakthrough cancer pain: dose titration versus proportional doses. Curr Med Res Opin 2012;28:963–8.ArticlePubMed
- 49. Yen TY, Chiou JF, Chiang WY, Su WH, Huang MY, Hu MH, et al. Proportional dose of rapid-onset opioid in breakthrough cancer pain management: an open-label, multicenter study. Medicine (Baltimore) 2018;97:e11593.ArticlePubMedPMC
- 50. Mercadante S, Maltoni M, Russo D, Adile C, Ferrera P, Rossi R, et al. The prevalence and characteristics of breakthrough cancer pain in patients receiving low doses of opioids for background pain. Cancers (Basel) 2021;13:1058.ArticlePubMedPMC
- 51. Passik SD, Kirsh KL. Weighing in on the off-label use of Actiq for noncancer-related pain: a recipe for success or a recipe for disaster? Pain Med 2007;8:130–3.ArticlePubMed
- 52. Mercadante S, Adile C, Cuomo A, Aielli F, Marinangeli F, Casuccio A. The use of low doses of a sublingual fentanyl formulation for breakthrough pain in patients receiving low doses of opioids. Support Care Cancer 2017;25:645–9.ArticlePubMedPDF
- 53. Preux C, Bertin M, Tarot A, Authier N, Pinol N, Brugnon D, et al. Prevalence of opioid use disorder among patients with cancer-related pain: a systematic review. J Clin Med 2022;11:1594.ArticlePubMedPMC
References
Figure & Data
References
Citations
Citations to this article as recorded by 

- Figure
- Related articles
-
- Home mechanical ventilation in children with chronic respiratory failure: a narrative review
- The mechanism of action of pulsed radiofrequency in reducing pain: a narrative review
- Beneficial effects of intermittent fasting: a narrative review
- Current diagnosis and treatment of vestibular neuritis: a narrative review

 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine
 PubReader
PubReader ePub Link
ePub Link Cite
Cite