PubMed Central, CAS, DOAJ, KCI

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Volume 40(Suppl); 2023 > Article
-
Resident fellow section: Clinical vignette
Cephalosporin-induced encephalopathy in patients with hematologic malignancies: a significant concern -
Young Seob Park1
 , Min Kyoung Kim1
, Min Kyoung Kim1 , Kyung Hee Lee1
, Kyung Hee Lee1 , Sung Ae Koh1
, Sung Ae Koh1 , Ji Yoon Jung1
, Ji Yoon Jung1 , Byeong Il Jang1
, Byeong Il Jang1 , Se-Jin Lee2
, Se-Jin Lee2
-
Journal of Yeungnam Medical Science 2023;40(Suppl):S137-S141.
DOI: https://doi.org/10.12701/jyms.2023.00864
Published online: November 14, 2023
1Department of Internal Medicine, Yeungnam University College of Medicine, Daegu, Korea
2Department of Neurology, Yeungnam University College of Medicine, Daegu, Korea
- Corresponding Author: Min Kyoung Kim, MD, PhD Department of Internal Medicine, Yeungnam University College of Medicine, 170 Hyeonchung-ro, Nam-gu, Daegu 42415, Korea Tel: +82-53-620-4683 • Fax: +82-53-654-8386 E-mail: kmk21c@med.yu.ac.kr
Copyright © 2023 Yeungnam University College of Medicine, Yeungnam University Institute of Medical Science
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 655 Views
- 28 Download
- A 73-year-old Korean woman visited the emergency room (ER) with symptoms of high fever and slight drowsiness. She had a history of intracerebral hemorrhage (ICH) diagnosed in 2008, with diabetes, hypertension, and chronic kidney disease (CKD). The patient had recovered from the ICH, and her motor function, sensory function, and level of consciousness were normal before admission. She was diagnosed with multiple myeloma (MM) 3 years previously. At that time, she was prescribed carfilzomib 27 mg/m2 intravenous (IV) on days 1, 2, 8, 9, 15, and 16; oral lenalidomide 10 mg on days 1 to 21; and dexamethasone 40 mg IV on days 1, 8, 15, and 22 as second-line treatment for MM for 8 months.
Patient information
- Immediately after arriving at the ER, her body temperature was 38.6°C, blood pressure was 90/60 mmHg, heart rate was 168 beats/min (newly diagnosed atrial fibrillation), and oxygen saturation was 97%. Accordingly, laboratory tests and blood, urine, and sputum cultures were performed. The Glasgow Coma Scale score was also assessed. The patient opened her eyes in response to sound, had a confused verbal response, and exhibited a localized motor response to pain. The patient scored 12 out of 15 points, indicating a mild drowsy state. Chest and abdomen auscultation and palpation were also performed. Apart from crackles heard during chest auscultation, no other significant abnormalities were detected.
Clinical findings
- In blood tests, the white blood cell count was 7,790 cells/µL (range, 4,000–10,000 cells/µL), and erythrocyte sedimentation rate and C-reactive protein (CRP) levels were elevated to 120 mm/hr (range, 0–25 mm/hr) and 15.2 mg/dL (range, 0–0.5 mg/dL), respectively. Moreover, the creatinine level was elevated to 3.52 mg/dL (range, 0.7–1.2 mg/dL), and creatinine clearance decreased to 12.72 mL/min (range, 75–125 mL/min). Chest computed tomography (CT) revealed multiple pulmonary consolidations. However, brain CT showed brain atrophy along with a previously implanted ventriculoperitoneal (VP) shunt. No acute brain lesions were observed that may have caused confusion. The patient exhibited severe general weakness, scoring 94 out of 150 on the manual muscle testing-8, and she was slightly drowsy. She was administered ceftazidime (2 g every 12 hours) until the 5th day of admission. After hospitalization, although the high fever improved, her level of consciousness deteriorated. On the 4th day of hospitalization, she became drowsy, and jerky movements were observed in her upper extremities and jaw.
Diagnostic assessment
- The following diagnoses were considered.
- 1. Uremic encephalopathy
- The patient already had CKD, and acute kidney injury had developed, further worsening her renal function. However, her urine output was not reduced and there was no acidosis or electrolyte imbalance, making it difficult to attribute encephalopathy to uremia.
- 2. Septic encephalopathy
- From the start of hospitalization, her blood pressure was maintained without the use of inotropic agents. The patient had an infection but did not experience septic shock. Furthermore, pyuria was not observed on the first day of admission, and no additional bacteria were identified in repeated cultures.
- 3. Cephalosporin-induced encephalopathy
- Despite worsening renal function, failure to reduce the antibiotic dose further may have contributed to cephalosporin-induced encephalopathy (CIE). In addition, the patient was 73 years old, and the blood-brain barrier damage resulting from ICH rendered her more vulnerable to CIE.
- 4. Cerebrovascular diseases (brain hemorrhage, brain infarction, etc.)
- The patient had a history of ICH. The penetration of cephalosporins into the central nervous system (CNS) may be increased in patients with prior brain injury. In fact, among patients with pre-existing CNS morbidity and CKD, the incidence of cefepime-induced neurotoxicity was approximately three times higher than that in patients with CKD but without previous CNS injury [1-3].
- 5. Multiple myeloma
- Encephalopathy directly caused by MM is rare. However, if MM is not well-controlled, a deteriorating general condition can indirectly lead to altered consciousness. Nevertheless, the patient maintained low levels of M-protein for several months before hospitalization, and although only a CT scan was performed, there were no signs of newly developed bone lesions suggestive of plasmacytoma.
Differential diagnosis
- Considering ICH history, the CNS was more vulnerable in the patient than in the general population, which significantly increased the likelihood of new brain-related issues emerging. Therefore, on the 6th day of hospitalization, brain magnetic resonance imaging (MRI) and follow-up brain CT were performed to assess the patient's condition. Although artifacts appeared secondary to the VP shunt valve on the brain MRI, no acute hemorrhage or ischemic findings were observed.
- The pneumonic consolidations improved over several days, during which time Escherichia coli was isolated from a urine culture. The patient had a high fever for the first 2 days. Her CRP level decreased from 15.2 mg/dL on the first day of admission to 5.8 mg/dL after 5 days. In the antibiotic susceptibility test, E. coli in the urine culture was confirmed to be sensitive to ceftazidime. Despite improvements in the patient’s fever, laboratory findings, and chest X-ray, there was no improvement in the patient’s level of consciousness. Therefore, it was challenging to conclude that sepsis was the primary cause of the decline in the patient’s consciousness. However, we adopted a proactive approach and transitioned from ceftazidime 2 g IV every 12 hours to meropenem 500 mg IV every 12 hours, considering the potential role of sepsis. Nevertheless, over several days, her level of consciousness deteriorated to a semi-comatose state with no voluntary movement and only a moan-like response to pain stimuli. Therefore, electroencephalography (EEG) was performed.
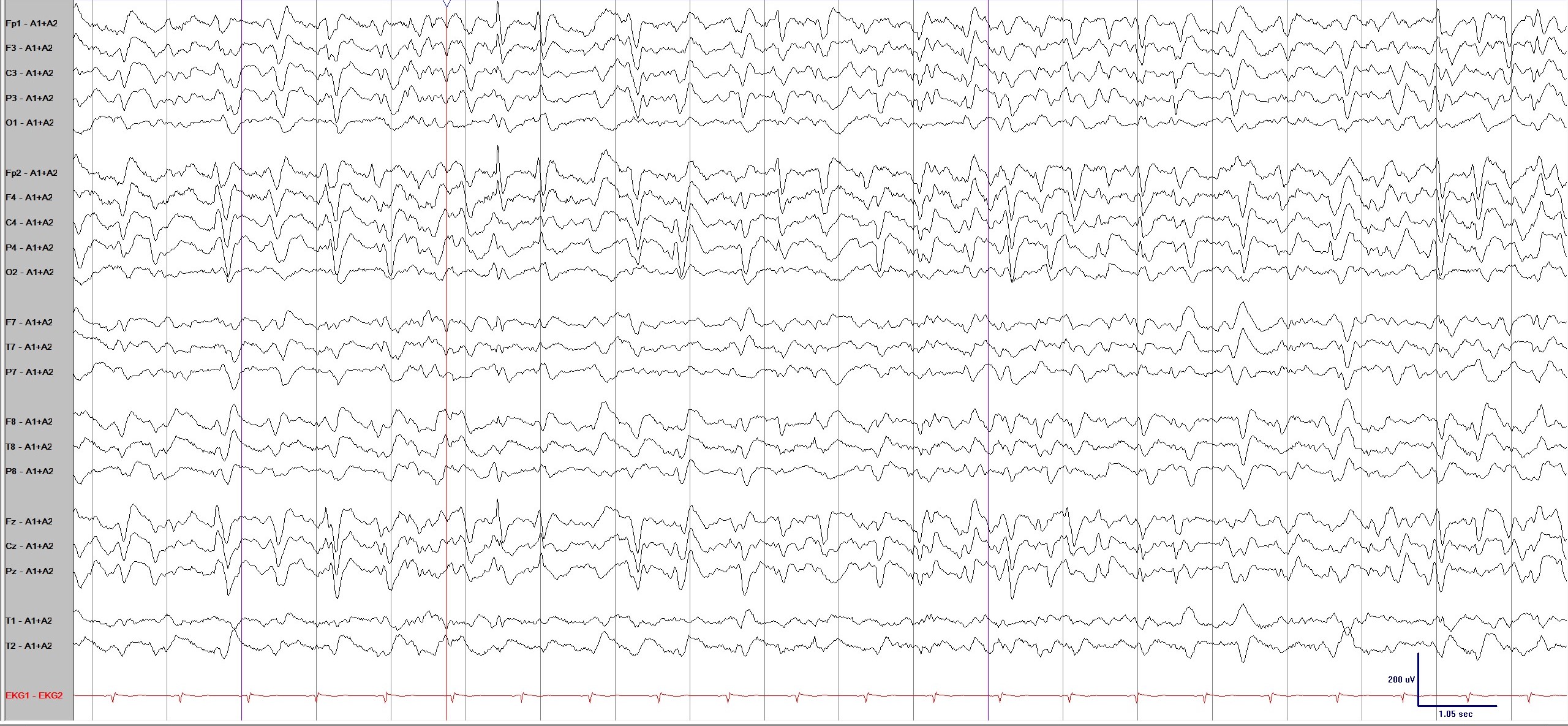
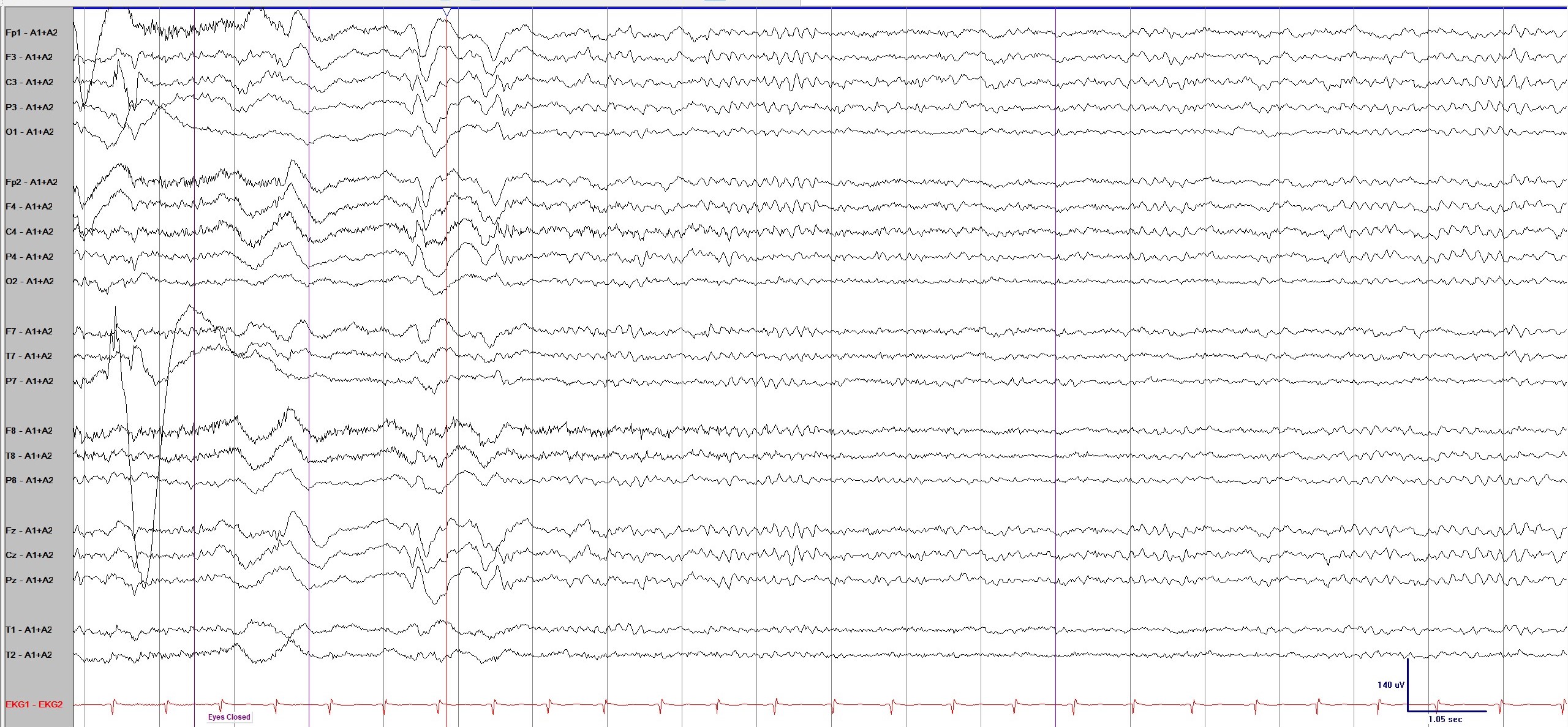
- The primary EEG (Fig. 1) revealed bilateral independent triphasic waves with slow background activity. This is an EEG finding commonly seen in CIE. Given that the likelihood of sepsis, uremia, and inherent brain problems as the causes of encephalopathy had decreased, the typical EEG findings of CIE raised the possibility that ceftazidime was the underlying cause. Three days after the discontinuation of ceftazidime, her level of consciousness gradually improved. A follow-up EEG (Fig. 2) was performed, and the last observed triphasic wave disappeared. After 3 days, a marked improvement in consciousness was observed. Two weeks after the discontinuation of ceftazidime, the patient recovered and was discharged.
Diagnosis and management
- Cephalosporins are frequently used in hospitals owing to their widespread antimicrobial activity. However, cephalosporins can produce several adverse effects including neurotoxicity [4]. CIE is primarily associated with cefepime use; therefore, its incidence is more specifically documented in patients administered cefepime. Among patients treated with cefepime, the prevalence of encephalopathy ranges from approximately 0.2% to 1%, with reported values as high as 7% in patients in intensive care units [1-3].
- However, the exact mechanism underlying CIE has not yet been definitively established. CIE is thought to be related to γ-aminobutyric acid (GABA) type A receptor inhibition through the competitive binding of cephalosporins. Cephalosporins may also decrease GABA release from the nerve terminals or increase excitatory amino acid release. Through these mechanisms, cefepime treatment results in hyperexcitation of neurons and depolarization of the postsynaptic membrane, which is clinically consistent with the occurrence of seizures, myoclonus, and encephalopathy [1,5-7].
- CIE is characterized by confusion, myoclonus, or seizures [5,6]. There are many risk factors for cephalosporin neurotoxicity, such as blood-brain barrier dysfunction due to preexisting CNS conditions, impaired renal function, old age, and excessive medication dosage [4,8].
- The EEG findings of CIE are generally consistent with those of metabolic encephalopathy, demonstrating generalized slowing and triphasic waves. Although these findings are not specific, they differ from EEG findings in patients with septic encephalopathy. During sepsis, various EEG findings are observed such as the absence of reactivity, slowing of background activity (theta and delta activity), electrophysiological seizures, and periodic discharges [8].
- The most effective treatment for CIE is the immediate discontinuation of cephalosporins. Although there are other treatments, such as hemodialysis and anticonvulsants, discontinuing cephalosporins is preferable. CIE is mostly reversible, and symptoms usually disappear within 7 days of discontinuation. In addition, to reduce neurotoxicity when cephalosporins are used, an appropriate dose based on renal function is vital [9].
Discussion
- Considering the extensive use of cephalosporins, CIE is considered a rare side effect. However, considering the significance of this class of antibiotics, internists should be cautious and carefully adjust the dosage according to renal function and various patient conditions when prescribing cephalosporins.
- Patients with hematological malignancies are inherently more susceptible to infections than general medical patients, leading to a significantly higher frequency of antibiotic use, including cephalosporins. Moreover, owing to the often-compromised renal function in these patients, they are more vulnerable to cephalosporin neurotoxicity than general medical patients. If neurological symptoms appear in patients with hematological malignancies, particularly MM, all possible causes should be considered. Among these, CIE is an important cause that should be assessed regardless of the type of cephalosporin used.
Conclusion
-
Ethical statements
This study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board (IRB) of the Yeungnam University Hospital (IRB No: 2023-08-014), and the requirement for informed consent was waived because of the retrospective nature of this study.
-
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
-
Funding
None.
-
Author contributions
Conceptualization, Data curation: all authors; Methodology, Supervision, Validation: YSP, MKK, SJL, KHL, SAK, JYJ, BIJ; Project administration, Visualization: YSP; Writing-original draft: YSP, MKK, SJL; Writing-review & editing: YSP, MKK, SJL, KHL, SAK, JYJ, BIJ.
Notes
- 1. Appa AA, Jain R, Rakita RM, Hakimian S, Pottinger PS. Characterizing cefepime neurotoxicity: a systematic review. Open Forum Infect Dis 2017;4:ofx170.ArticlePubMedPMCPDF
- 2. Fugate JE, Kalimullah EA, Hocker SE, Clark SL, Wijdicks EF, Rabinstein AA. Cefepime neurotoxicity in the intensive care unit: a cause of severe, underappreciated encephalopathy. Crit Care 2013;17:R264.ArticlePubMedPMCPDF
- 3. Kang JK, Kim SB. Neurotoxicity by cefepime: case-control study. J Neurocrit Care 2014;7:104–10.ArticlePDF
- 4. Lee SJ. Cefepime-induced neurotoxicity. J Neurocrit Care 2019;12:74–84.ArticlePDF
- 5. Al-Sadawi M, Rodriguez Ortega R, Sun N, Abdurahimova M, McFarlane SI. Jerky movement with ceftazidime: a case of ceftazidime-induced neurotoxicity with a review of the literature. Case Rep Med 2019;2019:8936478.ArticlePubMedPMCPDF
- 6. Keerty D, Shareef NA, Ramsakal A, Haynes E, Syed M. Cefepime-induced encephalopathy. Cureus 2021;13:e13125.ArticlePubMedPMC
- 7. Payne LE, Gagnon DJ, Riker RR, Seder DB, Glisic EK, Morris JG, et al. Cefepime-induced neurotoxicity: a systematic review. Crit Care 2017;21:276.ArticlePubMedPMCPDF
- 8. Triplett JD, Lawn ND, Chan J, Dunne JW. Cephalosporin-related neurotoxicity: metabolic encephalopathy or non-convulsive status epilepticus? J Clin Neurosci 2019;67:163–6.ArticlePubMed
- 9. Boschung-Pasquier L, Atkinson A, Kastner LK, Banholzer S, Haschke M, Buetti N, et al. Cefepime neurotoxicity: thresholds and risk factors. A retrospective cohort study. Clin Microbiol Infect 2020;26:333–9.ArticlePubMed
References
Figure & Data
References
Citations


 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine PubReader
PubReader ePub Link
ePub Link Cite
Cite



