PubMed Central, CAS, DOAJ, KCI

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Volume 40(Suppl); 2023 > Article
-
Case report
Classical Hodgkin lymphoma following follicular lymphoma: a case report -
Bomi Kim

-
Journal of Yeungnam Medical Science 2023;40(Suppl):S113-S122.
DOI: https://doi.org/10.12701/jyms.2023.00584
Published online: August 17, 2023
Department of Pathology, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
- Corresponding author: Bomi Kim, MD, PhD Department of Pathology, Inje University Haeundae Paik Hospital, Inje University College of Medicine, 875 Haeun-daero, Haeundae-gu, Busan 48108, Korea Tel: +82-51-797-3112 • Fax: +82-51-797-3101 • E-mail: domabem96@paik.ac.kr
Copyright © 2023 Yeungnam University College of Medicine, Yeungnam University Institute of Medical Science
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,685 Views
- 107 Download
Abstract
- The simultaneous, composite, or sequential occurrence of follicular lymphoma (FL) and classical Hodgkin lymphoma (HL), both of which originate from germinal center B-cell, is rare. Questions have been raised with regard to the type of tests that pathologists should perform when observing the presence of a “large-cell lymphoma” following an FL and what are the most critical pathological points for diagnosis. Here, we present a case of a classical HL following an FL after administering rituximab-bendamustine (R-Benda) chemotherapy. Furthermore, we also summarized the literature and compared this case with other HLs that followed FLs. A 55-year-old woman was diagnosed with a grade 3A FL of the breast and axillary lymph node masses. She completed six R-Benda chemotherapy cycles for stage IV FL. Twenty-three months after the diagnosis, follow-up image studies showed an increase in the size and number of the lesions. Biopsies of the neck lymph node and liver were performed, and the diagnosis was classical HL. Sequential or composite FL and HL may sometimes develop from the same clone because they share the same genetic alterations, such as B-cell lymphoma (Bcl)-2 or Bcl-6 translocation. When a large-cell lymphoma is found after the treatment of FL, classical HL should be considered a pathological differential diagnosis, and histological, immunohistochemical, or molecular investigations must be considered during the diagnostic process.
- Clinicians and pathologists often encounter secondary tumors after the treatment of primary tumors. In malignant lymphomas, it is well known that low-grade lymphomas such as follicular lymphomas (FLs) can be transformed into high-grade, diffuse large B-cell lymphomas (DLBCLs) [1]. Although Hodgkin lymphoma (HL) and non-HL are different types of malignant lymphoma, reports on “consequent” lymphomas are rare [2]. These tumors, often found in breast or prostate cancers, should be differentiated from lymphomas that changed their morphological findings due to the administration of treatment. Thus, which tests should pathologists perform when observing the presence of a “large-cell lymphoma” following a FL? What are the most critical pathological points for diagnosis? Here, we present a case of a classical HL that developed following rituximab-bendamustine (R-Benda) chemotherapy for FL treatment. We also summarized the literature and compared this case with other HLs that followed FLs.
Introduction
- Ethical statements: This study followed the recommendations of the World Medical Association Declaration of Helsinki and was approved by Institutional Review Board (IRB) of Inje University Haeundae Paik Hospital in Busan, Korea (IRB No: 2021-01-009). The need for informed consent was waived due to the retrospective nature of the study.
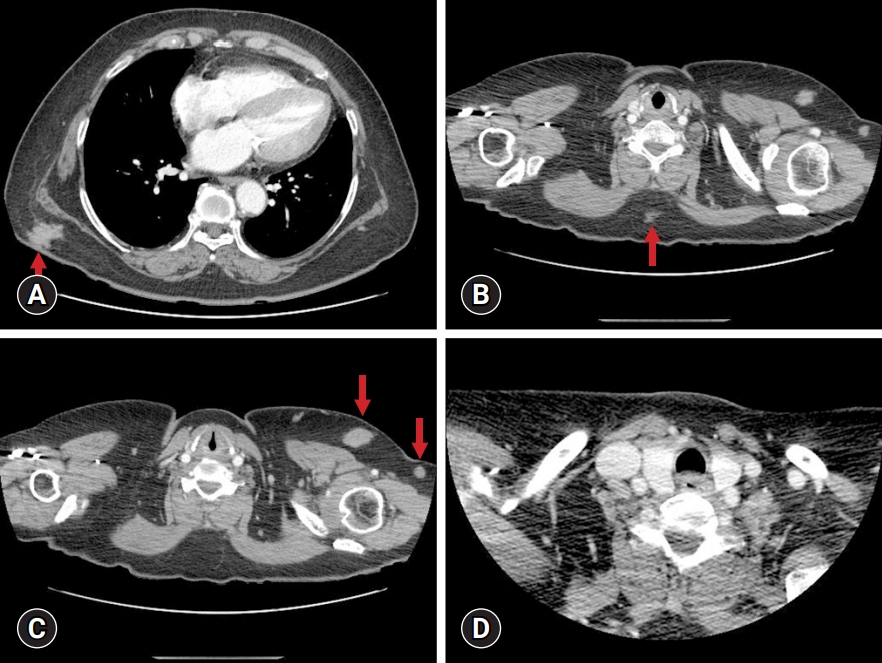
- A 55-year-old Asian woman presented with a painful mass on the left anterior chest wall. She was being treated for hypertension, hyperlipidemia, diabetes mellitus, and rheumatoid arthritis. Chest computed tomography (CT) revealed multiple subcutaneous enhancing masses on the left breast, right lower back, and mid-back, measuring between 12 and 26 mm (Fig. 1A–1C). The axillary lymph nodes on the left side were enlarged (Fig. 1B, 1C). The neck CT was normal (Fig. 1D). Excisional biopsies of the left breast and the solid axillary masses were performed.
- A low-power view of the breast lesion showed an abnormal follicular growth pattern, which did not have tingible body macrophages or well-developed mantle zones (Fig. 2A). The abnormal follicular pattern was also found in the extranodal lymphoid adipose tissue through lymphoid capsules (Fig. 2B), with neoplastic follicles composed of centroblasts (Fig. 2C). However, Reed-Sternberg, Hodgkin cells, or popcorn cells were absent. The CD21 immunohistochemistry highlighted follicular dendritic cell meshworks, including an abnormal structure of the adipose tissue (Fig. 2D). The CD20 (Fig 2E) and B-cell lymphoma (Bcl)-6 antibodies (Fig. 2F) reacted to the neoplastic follicles. Sparse CD10-positive cells were observed in neoplastic follicles (Fig 2G). Moreover, the Bcl-2 immunohistochemistry showed a variable extent of positivity in neoplastic follicles (Fig. 2H), and some follicles were positive for Bcl-2 immunohistochemistry, with a diffuse or weakly patchy pattern (Fig. 2H). The expression of CD10, CD5, MUM1, and cyclin D1 was negative, and CD3-positive intrafollicular T cells were also found in neoplastic follicles. The Ki-67 labeling index was as high as 50% (Fig. 2I). Thus, the histological findings of the axillary masses were identical to those of the breast. The diagnosis was FL, grade 3A, with a follicular pattern.
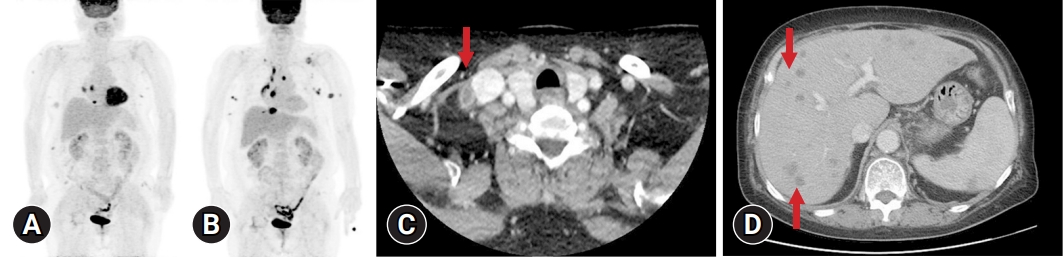
- The positron emission tomography (PET) for staging workup revealed multiple subcutaneous nodules of the shoulder, back, flank, breast, and axilla and enlarged lymph nodes of the left axilla, anterior diaphragmatic, and right interlobar areas (Fig. 3A). The bone marrow test was negative for malignant lymphoma. Although chemotherapy was recommended for stage IV FL, the patient refused it and visited a clinic for regular radiological studies. After 17 months of FL, the PET revealed new lesions of the supraclavicular, mediastinal, and left axillary lymph nodes and increased size of the anterior diaphragmatic lesions, despite no evidence of progression in previous image tests (Fig. 3B). The patient completed six cycles of R-Benda. Twenty-three months later, after the first diagnosis of FL, during regular image check-ups, such as CT scans of the neck, chest, abdomen, and pelvis and PET scans, the patient was examined for multiple nodules that may have made a new appearance in the right supraclavicular (Fig. 3C), mediastinal, and abdominal cavity lymph nodes, as well as in the liver (Fig. 3D), spleen, or bilateral lungs, which is consistent with progressive disease. An incisional biopsy of the right supraclavicular lymph node and a needle biopsy of the mass in segment 5/8 of the liver were performed to confirm the diagnosis.
- At a low magnification of the supraclavicular lymph node specimen, the normal lymph node architecture was replaced by the proliferation of large histiocytoid cells with extensive necrosis (Fig. 4A) with the Reed-Sternberg cells being rarely observed in the background of the histiocytes and sparse lymphocytes (Fig. 4B, 4C). No follicular growth pattern and only a small number of lymphoid cells were observed. The Reed-Sternberg cells were positive for CD30 (Fig. 4D), PAX-5 (Fig. 4E), MUM1(Fig. 4F), and Bcl-2. Furthermore, the cells were negative for CD20, CD79a, CD15, anaplastic lymphoma kinase (ALK; also known as CD236), CD10, and cyclin D1, and the Bcl-6 antibodies reacted weakly to these cells (Fig. 4G). The in situ hybridization of the Epstein-Barr virus-encoded small RNAs (EBER ISH) was negative. The Bcl-2 translocation fluorescence in situ hybridization (FISH) using a Vysis Bcl-2 dual color, break-apart rearrangement probe (Abbott Molecular, Des Plaines, IL, USA) was negative for the Bcl-2 (18q21) translocation or copy number gain/amplification. The diagnosis was classical HL, an unclassifiable subtype, although some parts of the lesion resembled classical HL, a lymphocyte-depleted subtype.
- The liver exhibited similar pathologic findings to those of the neck lymph nodes. The hepatic lesion displayed large cells accompanied by fibrosis, clearly distinguishable from the surrounding non-neoplastic hepatic parenchyma (Fig. 4H). Notably, the presence of Reed-Sternberg cells and Hodgkin cells was more frequent compared to the lymph node biopsy (Fig. 4I), with positive immunostaining observed for CD30, PAX-5, Bcl-6, Bcl-2, and MUM1. The Ki-67 labeling index was low, primarily expressed in Reed-Sternberg cells. Negative results were obtained for CD20, CD79a, ALK (CD246), and cyclin D1, as well as EBER ISH. Additionally, the Bcl-2 translocation FISH yielded negative results for Bcl-2 (18q21) translocation or copy number gain/amplification. The polymerase chain reaction (PCR) analysis for clonal immunoglobulin heavy chain (IgH) gene rearrangements was inconclusive, displaying a peak near the cutoff within the valid size range. Thus, the unclassifiable classical HL diagnosis was established for liver involvement as well.
Case
- Despite the diagnostic limitations due to the impossibility of assessing all tissues of the first and second-time diagnosis, it is possible to state that the composite lymphoma of FL and classical HL recurred as a form of classical HL. To minimize diagnostic error, the submitted tissues were entirely embedded and reviewed several times. To rule out nodular lymphocyte-predominant HL, we reexamined the diagnosed FL and found no large tumor cells such as popcorn cells. In addition, large lymphoid cells of the supraclavicular and liver biopsies were positive for CD30 and negative for CD20, supporting the diagnosis of classical HL. Moreover, no T-cell rosettes were found. Large B cell lymphoma with interferon regulatory factor rearrangement was also ruled out because of MUM1 immunohistochemistry negativity. Here, we confirmed that pathological findings were quite different between the first and the second diagnoses, with the second and the third lesion appearing after the completion of the treatment.
- Simultaneous, composite, or sequential occurrence of FL and classical HL, which both originate from germinal center B-cell, is rare. Compared with classical HLs, nodular lymphocyte-predominant HLs are associated more commonly with B-cell lymphoma. With regard to classical HL following FL, as in this case, Jaffe et al. [2] reported that lymphomas of HLs following FLs are the most frequent consequent lymphomas. This may have been due to the high incidence of FLs in Western countries. Notably, FLs do not occur in East Asia as often as they do in Western countries [3]. We have compiled 35 cases of classical HLs preceded by FLs by reviewing several original articles [4-17] (Table 1). Moreover, nine cases in China and Japan have been reported since 1996. Considering that the incidence of FL in South Korea is rising, it is important to report this case that occurred in our country [18].
- HLs following FLs appeared in patients aged 27 to 89 years, and the time interval between the FLs and HLs ranged from 12 to 276 months. The HLs were observed as composite (two cases), simultaneous (three cases), or both composite and simultaneous (three cases), as well as pure classical HL (27 cases). It is generally accepted that FLs and HLs originate from transformed mutating and antigen-selected germinal center B-cells and preapoptotic germinal B-cells, respectively [19]. Some composite or sequential lymphomas exhibited shared genetic alterations, including translocation involving Bcl-2 or Bcl-6, or clonality of IgH or immunoglobulin kappa chain rearrangement These findings suggest neoplastic transdifferentiation occurring between the different components of the two lymphomas [20]. Trecourt et al. [20] reported that mutations in BCL2, CREBBP, KMT2D, EP300, and ARID1A are frequently observed in both sequential and composite lymphomas of FL and HL. These mutations, which are found usually in FLs but not in HLs, may serve as driver mutations in the development of these lymphomas. However, different mutations specific to each contingent may have been secondary or passenger mutations [20]. Thus, it is plausible that this particular case also exhibited genetic alterations shared between FLs and HLs, although these specific alterations were not assessed in this study.
- In our cases, we considered the pathological diagnosis to be an anaplastic variant of DLBCL, HL, anaplastic large-cell lymphoma, or FL showing anaplastic large-cell lymphoma-like features with a treatment effect. The negative result for both CD20 and CD79a ruled out the diagnosis of B-cell lymphoma, and a low Ki-67 labeling index eliminated the possibility of DLBCL. Furthermore, an anaplastic large-cell lymphoma was excluded based on the PAX-5 expression. Given that the IgH rearrangement clonality and BCL2 gene translocation may sometimes be found in both HLs and FLs of consequent lymphoma [10,14-17,20], pathologists should not rely on them for molecular investigations. While HLs and FLs have been shown to have different peaks in IgH gene rearrangement PCR, they were observed to have IgH clonality [17]. Notably, the most crucial point was using a basic morphologic assessment, such as assessing the Reed-Sternberg cells, background cells, and growth patterns.
- We presented a case of HL that occurred 23 months after FL. The FL and HL originated from germinal center B-cells. Sequential or composite FL and HL sometimes share common genetic alterations, which supports the idea that they shared identical clones and that transdifferentiation may cause composite or sequential lymphomas. Clinical, histological, immunohistochemical, and molecular investigations are critical for a correct diagnosis.
Discussion
-
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
-
Funding
None.
Notes


| No. | Case | Year | Age at initial diagnosis (yr)/sex | Initial diagnosis (site) | Initial therapy/response | Interval to transformation (time of relapse, mo) | Second diagnosis (site) | Second therapy/response | Follow-up from the initial diagnosis (mo) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Custer and Bernhard [4] | 1948 | 33/F (case 1) | FL (LN) | RT/NA | NA | HL (LN) | NA | 23 | DOD |
| 2 | Custer and Bernhard [4] | 1948 | NA | FL (LN) | NA/NA | NA | HL (LN) | NA | NA | DOD |
| 3 | Carrato et al. [5] | 1987 | 68/F (case 2) | FL CCC (neck LN) | RT /CR | 60 | HL NS (left axillary LN, supraclavicular, portahepatic, paraaortic, peripancreatic, and mesenteric nodes; spleen; liver; left lung; left kidney; and BM) | Vinblastine, thiotepa, procarbazine, and prednisone+palliative RT/NC | 62 | DOD |
| 4 | Carrato et al. [5] | 1987 | 40/M (case 3) | FL CCC (right submandibular and left inguinal LN) | RT, chlorambucil/CR | 276 | HL NS (right inguinal node) | Cyclophosphamide, vincristine, procarbazine, and prednisone, (COPP) | 354 | NED |
| FL CCC or mantle cell lymphoma (left inguinal LN) | → lomustine, vinblastine, procarbazine, and prednisone | |||||||||
| → prednisone, lomustine, vinblastine, and procarbazine | ||||||||||
| → lomustine, vinblastine, procarbazine, prednisone, and methotrexate/PD | ||||||||||
| →CR | ||||||||||
| 5 | Carrato et al. [5] | 1987 | 74/M (case 5) | FL CCC (small bowel, retroperitoneum) | Surgery, RT (3500 rad) vincristine, cyclophosphamide, and chlorambucil/CR | 92 | Simultaneous: | MOPP-ABV-CAD/CR | 120 | NED |
| HL NS (left neck LN) | ||||||||||
| FL CCC (BM) | ||||||||||
| 6 | Lenner et al. [6] | 1989 | 39/M | FL CCC and CBC (inguinal LN) | Oral prednomustine/CR | 36 | Composite: | Alternating MOPP and AVBD/PD | 72 | AWD |
| HL MC and FL CCC and CBC (spleen, hepatoduodenal, paraaortic and inguinal LNs) | ||||||||||
| 7 | Gonzalez et al. [7] | 1991 | 43/M (case 2, case 3 of Jaffe 1992) | DLBCL (NA) | NA/NA | 48 | Simultaneous and composite | CT, AMBT/PD | 26 | DOD |
| FL CCC and CBC (NA) | HL UC, FL and DLBCL (inguinal LN) | |||||||||
| HL NS and DLBCL (90%) (supraclavicular LN) | ||||||||||
| 8 | Gonzalez et al. [7] | 1991 | 63/M (case 3) | FL CCC and CBC (NA) | NA | 36 | Composite: | Surgery | 2 | DOD |
| HL MC (10%) and DLBCL (90%) (stomach) | ||||||||||
| 9 | Gonzalez et al. [7] | 1991 | 48/M (case 8, case 1 of Jaffe 1992) | FL CCC and CBC (NA) | NA | 144 | Composite: | RT, nitrogen mustard, vincristine, prednisone, procarbazine/PD | 35 | DOD |
| HL NS and FL CCC and CBC (large cell 75%) (neck and inguinal LN) | ||||||||||
| HL interfollicular type and FL CCC and CBC (large cell 90%) (submandibular LN) | ||||||||||
| 10 | Travis et al. [8] | 1992 | 39/F (case 5) | FL CCC (NA) | CT | 65 | HL UC (LN) | NA | 69 | DOC |
| 11 | Travis et al. [8] | 1992 | 57/F (case 6) | FL CCC and CBC (LN) | NA | 137 | HL NS (LN and BM) | NA | 141 | DOC |
| 12 | Zarate-Osorno et al. [9] | 1993 | 27/F (case 1) | FL CCC and CBC (supraclavicular LN) | C-MOPP, CHOP, RT | 96 | HL NS (subxiphoid LN) | CT | 114 | DOD |
| 13 | Zarate-Osorno et al. [9] | 1993 | 61/F (case 2) | FL CCC and CBC (neck LN) | Cyclophosphamide, prednisone, vincristine, procarbazine | 48 | HL NS (supraclavicular LN) | Cyclophosphamide, vincristine, prednisone, etoposide | 66 | NED |
| 14 | Zarate-Osorno et al. [9] | 1993 | 72/M (case 3) | FL CBC (neck and intra-auricular LN) | Cyclophosphamide, prednisone, vincristine, RT | 36 | HL NS (inguinal LN) | RT | 70 | NED |
| 15 | Zarate-Osorno et al. [9] | 1993 | 57/F (case 4) | FL CCC (inguinal LN) | Prednisone, chlorambucil | 144 | HL UC (periaortic LN, liver) | ABVD/MOPP, etoposide | 153 | NED |
| 16 | Zarate-Osorno [9] | 1993 | 54/F (case 5) | FL CCC and CBC (femoral LN) | RT, chlorambucil, prednisone | 60 | HL NS (neck LN) | CT | 60 | NA |
| 17 | Zarate-Osorno et al. [9] | 1993 | 36/M (case 7) | FL CCC (inguinal and axillary LN) | None | 24 | Simultaneous: | ProMACE-MOPP, RT, AMBT | 60 | DOD |
| HL MC (inguinal LN) | ||||||||||
| FL CCC (inguinal LN) | ||||||||||
| 18 | Zarate-Osorno et al. [9] | 1993 | 36/F (case 8) | FL and DLBCL cell (Jejunum) | ProMACE-MOPP | 72 | Simultaneous and composite: | ProMACE | 150 | NED |
| FL, CCC (mesenteric LN) | HL NS (periaortic LN) | CytaBOM RT | ||||||||
| FL and DLBCL (mesenteric LN) | ||||||||||
| 19 | LeBrun et al. [10] | 1994 | NA (HH) | FL CBC (NA) | RT | 180 | HL NS (NA) t (18;14)(+) | NA | NA | NA |
| 20 | LeBrun et al. [10] | 1994 | NA (HN) | FL CCC (NA) | RT | 132 | HL MC (NA) t (18;14)(+) | NA | NA | NA |
| 21 | LeBrun et al. [10] | 1994 | NA (VS) | FL CCC (NA) | CT | 48 | HL NS (NA) t (18;14)(–) | NA | NA | NA |
| 22 | LeBrun et al. [10] | 1994 | NA (BB) | FL CCC (NA) | NA | 84 | HL MC (NA) | NA | NA | NA |
| 23 | LeBrun et al. [10] | 1994 | NA (AL) | FL CCC (NA) | NA | 84 | HL MC (NA) | NA | NA | NA |
| 24 | LeBrun et al. [10] | 1994 | NA (LD) | FL CCC (NA) | NA | 12 | HL UC (NA) | NA | NA | NA |
| 25 | Hirose et al. [11] | 1996 | 62/M | FL CCC (neck LN, duodenum) | CHOP #5/PD | 16 | Simultaneous: | Surgery, CT | 18 | DOC |
| EBER(–) | HL MC (inguinal, paraaortic, mesenteric, and along the lesser curvature LN) | |||||||||
| EBER(+) | ||||||||||
| FL CCC (duodenum) | ||||||||||
| 26 | Thirumala et al. [12] | 2000 | 60/M | FL, CCC (unspecified site) | RT | 156 | Composite: | NA | NA | NA |
| HL NS and FL CCC (inguinal LN) | ||||||||||
| 27 | Copur et al. [13] | 2004 | 68/F | FL CCC (10%) and CLL (90%) (BM) | SNOP #6 | 96 | HL (liver and retroperitoneal LN) | Methylprednisolone | NA | NA |
| → FL (BM) | →fludarabine #6, rituximab #4/PR | t(14;18)(–) | → Liposomal doxorubicin #4/CR | |||||||
| FL CCC (inguinal, mesenteric, periaortic, pericaval, and liver) | →PD→CR | |||||||||
| 28 | Nakamura et al. [14] | 2007 | 44/M | FL grade 1 (abdominal cavity) | NA | 48 | HL MC (neck LN) | NA | NA | NA |
| EBER(–), t(14;18)(+) | EBER(–), t(14;18)(+) | |||||||||
| 29 | Yoshida et al. [15] | 2012 | 63/M (case 3) | FL, grade 3A (inguinal LN) | NA | 120 | HL MC (systemic) EBER(–), translocation of 18q21 (+) | NA | NA | NA |
| 30 | Yoshida et al. [15] | 2012 | 89/F (case 4) | FL. grade 1 (colon) | NA | 36 | HL MC (neck LN) | NA | NA | NA |
| EBER(–), t(14;18)(+) | t(14;18)(+) | |||||||||
| 31 | Wang et al. [16] | 2016 | 63/M (case 2) | FL (site unspecified), stage IV | R-CVP #6/PD | 72 | HL (paraaortic and retroperitoneal LNs) | NA/NA | NA | NA |
| 32 | Wang et al. [16] | 2016 | 53/M (case 3) | FL grade 3, stage IV | R-CHOP #8/CR | 66 | HL NS (mediastinal LN) | CT #2, Auto-SCT/PD | 0 | DOD |
| t(14;18)(+) | t(14;18)(+) | |||||||||
| FL grade 2 (lung) | t-MDS (BM) | |||||||||
| 33 | Wang et al. [16] | 2016 | 48/M (case 4) | FL grade 1 (inguinal LN) | R-Benda #4 | 24 | HL NS (paraaortic, retroperitoneal, peribiliary LNs) | None | 0 | DOC |
| IgH(+) t(14;18)(+ ) | R-CHOP #6/PD | IgH+ t(14;18)+ | ||||||||
| 34 | Wang et al. [16] | 2016 | 68/M (case 5) | FL grade 1–2 (inguinal LN) IgH(+) | R-Benda ibritumomab tiuxetan/PD | 12 | Composite: HL NS (axillary LN) | ABVD #3 | 48 | AWD |
| IgH(+) t(14;18)(+) | → brentuximab and then gemcitabine, vinorelbine, and doxorubicin | |||||||||
| FL low grade (inguinal LN) | → RICE → brentuximab | |||||||||
| → ipilimumab and nivolumab/PD | ||||||||||
| 35 | Tennese et al. [17] | 2017 | 49/M | FL, grade 3A, SLL, and MCLIS (left inguinal LN) | CHOP #6 → fludarabine, chlorambucil, and rituximab → autologous stem cell transplant/PR → CR | 144 | HL MC (right axillary LN) | ABVD #4 | 168 | DOC (sepsis) |
| EBER(–) IgH(+) t(14;18)(+) in FL | EBER(–) IgH(+), a different peak from previous FL | →gemcitabine and vinorelbine/CR | ||||||||
| 14q32/IgH translocation in SLL | →PD | |||||||||
| CCND1/IgH translocation in MCLIS | ||||||||||
| FL grade 1–2, SLL (submental LN) |
F, female; FL, follicular lymphoma; LN, lymph node; RT, radiotherapy; NA, not applicable; DOD, died of disease; CCC, centrocytic type; NS, nodular sclerosing; NC, non-curative; M, male; CR, complete remission; COPP, cyclophosphamide, oncovin, procarbazine, prednisone; PD, progressive disease; NED, no evidence of disease; MOPP, nitrogen mustard, vincristine, procarbazine, prednisone; ABV, adriamycin (doxorubicin), bleomycin, vinblastine; CAD, comustine, alkeram, diacetylvinblastine (vindesine); MC, mixed cellularity; AVBD, adriamycin (doxorubicin), bleomycin, vinblastine, dacarbazine; AWD, alive with disease; DLBCL, diffuse large B-cell lymphoma; CBC, centroblastic type; UC, unclassifiable; CT, computed tomography; DOC, died of complications or died of other cause; BM, bone marrow; AMBT, autologous bone marrow transplantation; ProMACE-MOP, prednisone, methotrexate, adriamycin (doxorubicin), cyclophosphamide, etoposide-mustargen (mechlorethamine), oncovin (vincristine), procarbazine, prednisone; CytaBOM, cytarabine, bleomycin, vincristine (oncovine), methotrexate; EBER, Epstein-Barr virus (EBV)-encoded small RNA; CLL, chronic lymphocytic leukemia; SNOP, cyclophosphamide, mitoxantrone, vincristine, prednisone; PR, partial response; R-CVP, rituximab, cyclophosphamide, vincristine, prednisolone; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; t-MDS, therapy-related myelodysplastic syndrome; SCT, stem cell transplantation; IgH, immunoglobulin heavy chain; R-Benda, rituximab-bendamustine; RICE, rituximab, ifosfamide, carboplatin, etoposide; SLL, small lymphocytic lymphoma; MCLIS, mantle cell lymphoma in situ; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; CCND1, cyclin D1.
- 1. Lossos IS, Gascoyne RD. Transformation of follicular lymphoma. Best Pract Res Clin Haematol 2011;24:147–63.ArticlePubMedPMC
- 2. Jaffe ES, Zarate-Osorno A, Medeiros LJ. The interrelationship of Hodgkin’s disease and non-Hodgkin’s lymphomas: lessons learned from composite and sequential malignancies. Semin Diagn Pathol 1992;9:297–303.ArticlePubMed
- 3. Huh J. Epidemiologic overview of malignant lymphoma. Korean J Hematol 2012;47:92–104.ArticlePubMedPMC
- 4. Custer RP, Bernhard WG. The interrelationship of Hodgkin’s disease and other lymphatic tumors. Am J Med Sci 1948;216:625–42.ArticlePubMed
- 5. Carrato A, Filippa D, Koziner B. Hodgkin’s disease after treatment of non-Hodgkin’s lymphoma. Cancer 1987;60:887–96.ArticlePubMed
- 6. Lenner P, Roos G, Hedenus M, Lindh J. Simultaneous presentation of relapsing non-Hodgkin’s lymphoma and Hodgkin’s disease. Eur J Haematol 1989;42:315–6.ArticlePubMed
- 7. Gonzalez CL, Medeiros LJ, Jaffe ES. Composite lymphoma: a clinicopathologic analysis of nine patients with Hodgkin’s disease and B-cell non-Hodgkin’s lymphoma. Am J Clin Pathol 1991;96:81–9.ArticlePubMed
- 8. Travis LB, Gonzalez CL, Hankey BF, Jaffe ES. Hodgkin’s disease following non-Hodgkin’s lymphoma. Cancer 1992;69:2337–42.ArticlePubMed
- 9. Zarate-Osorno A, Medeiros LJ, Kingma DW, Longo DL, Jaffe ES. Hodgkin’s disease following non-Hodgkin’s lymphoma: a clinicopathologic and immunophenotypic study of nine cases. Am J Surg Pathol 1993;17:123–32.ArticlePubMed
- 10. LeBrun DP, Ngan BY, Weiss LM, Huie P, Warnke RA, Cleary ML. The bcl-2 oncogene in Hodgkin’s disease arising in the setting of follicular non-Hodgkin’s lymphoma. Blood 1994;83:223–30.ArticlePubMedPDF
- 11. Hirose Y, Iwabuchi K, Shimizu S, Sasaki K, Nojima T, Takiguchi T. Nodal EBV-positive Hodgkin’s disease following extranodal EBV negative non-Hodgkin’s lymphoma of B-cell lineage. Eur J Haematol 1996;57:103–6.ArticlePubMed
- 12. Thirumala S, Esposito M, Fuchs A. An unusual variant of composite lymphoma: a short case report and review of the literature. Arch Pathol Lab Med 2000;124:1376–8.ArticlePubMed
- 13. Copur MS, Ledakis P, Novinski D, Fu K, Hutchins M, Frankforter S, et al. An unusual case of composite lymphoma involving chronic lymphocytic leukemia follicular lymphoma and Hodgkin disease. Leuk Lymphoma 2004;45:1071–6.ArticlePubMed
- 14. Nakamura N, Ohshima K, Abe M, Osamura Y. Demonstration of chimeric DNA of bcl-2 and immunoglobulin heavy chain in follicular lymphoma and subsequent Hodgkin lymphoma from the same patient. J Clin Exp Hematop 2007;47:9–13.ArticlePubMed
- 15. Yoshida M, Ichikawa A, Miyoshi H, Takeuchi M, Kimura Y, Nino D, et al. High frequency of t(14;18) in Hodgkin’s lymphoma associated with follicular lymphoma. Pathol Int 2012;62:518–24.ArticlePubMed
- 16. Wang XJ, Griffin GK, Yenamandra A, Wheeler FC, Ligon AH, Nandedka MA, et al. Transformation of follicular lymphoma into classical Hodgkin lymphoma showing t(14;18). Hematopathology 2016;1:23–33.
- 17. Tennese A, Skrabek PJ, Nasr MR, Sekiguchi DR, Morales C, Brown TC, et al. Four lymphomas in 1 patient: a unique case of triple composite non-Hodgkin lymphoma followed by classical Hodgkin lymphoma. Int J Surg Pathol 2017;25:276–80.ArticlePubMedPDF
- 18. Kim M, Hwang HS, Cho H, Yoon DH, Suh C, Park CS, et al. Upward trend in follicular lymphoma among the Korean population: 10-year experience at a large tertiary institution. J Pathol Transl Med 2021;55:330–7.ArticlePubMedPMCPDF
- 19. Küppers R, Sousa AB, Baur AS, Strickler JG, Rajewsky K, Hansmann ML. Common germinal-center B-cell origin of the malignant cells in two composite lymphomas, involving classical Hodgkin’s disease and either follicular lymphoma or B-CLL. Mol Med 2001;7:285–92.ArticlePubMedPMCPDF
- 20. Trecourt A, Mauduit C, Szablewski V, Fontaine J, Balme B, Donzel M, et al. Plasticity of mature B cells between follicular and classic Hodgkin lymphomas: a series of 22 cases expanding the spectrum of transdifferentiation. Am J Surg Pathol 2022;46:58–70.ArticlePubMed
References
Figure & Data
References
Citations


 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine PubReader
PubReader ePub Link
ePub Link Cite
Cite





