PubMed Central, CAS, DOAJ, KCI

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Volume 40(4); 2023 > Article
-
Original article
Diagnostic value of serum procalcitonin and C-reactive protein in discriminating between bacterial and nonbacterial colitis: a retrospective study -
Jae Yong Lee*
 , So Yeon Lee*
, So Yeon Lee* , Yoo Jin Lee
, Yoo Jin Lee , Jin Wook Lee
, Jin Wook Lee , Jeong Seok Kim
, Jeong Seok Kim , Ju Yup Lee
, Ju Yup Lee , Byoung Kuk Jang
, Byoung Kuk Jang , Woo Jin Chung
, Woo Jin Chung , Kwang Bum Cho
, Kwang Bum Cho , Jae Seok Hwang
, Jae Seok Hwang
-
Journal of Yeungnam Medical Science 2023;40(4):388-393.
DOI: https://doi.org/10.12701/jyms.2023.00059
Published online: April 3, 2023
Division of Gastroenterology, Department of Internal Medicine, Keimyung University Dongsan Medical Center, Keimyung University School of Medicine, Daegu, Korea
- Corresponding author: Yoo Jin Lee, MD Division of Gastroenterology, Department of Internal Medicine, Keimyung University School of Medicine, 1035 Dalgubeol-daero, Dalseo-gu, Daegu 42601, Korea Tel: +82-53-258-7739 • Fax: +82-53-258-4341 • E-mail: doctorlyj@dsmc.or.kr
- *These authors contributed equally to this work.
•This study was presented through a poster presentation at Korea Digestive Disease Week (KDDW) 2021.
Copyright © 2023 Yeungnam University College of Medicine, Yeungnam University Institute of Medical Science
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,227 Views
- 52 Download
Abstract
-
Background
- Differentiating between bacterial and nonbacterial colitis remains a challenge. We aimed to evaluate the value of serum procalcitonin (PCT) and C-reactive protein (CRP) in differentiating between bacterial and nonbacterial colitis.
-
Methods
- Adult patients with three or more episodes of watery diarrhea and colitis symptoms within 14 days of a hospital visit were eligible for this study. The patients’ stool pathogen polymerase chain reaction (PCR) testing results, serum PCT levels, and serum CRP levels were analyzed retrospectively. Patients were divided into bacterial and nonbacterial colitis groups according to their PCR. The laboratory data were compared between the two groups. The area under the receiver operating characteristic curve (AUC) was used to evaluate diagnostic accuracy.
-
Results
- In total, 636 patients were included; 186 in the bacterial colitis group and 450 in the nonbacterial colitis group. In the bacterial colitis group, Clostridium perfringens was the commonest pathogen (n=70), followed by Clostridium difficile toxin B (n=60). The AUC for PCT and CRP was 0.557 and 0.567, respectively, indicating poor discrimination. The sensitivity and specificity for diagnosing bacterial colitis were 54.8% and 52.6% for PCT, and 52.2% and 54.2% for CRP, respectively. Combining PCT and CRP measurements did not increase the discrimination performance (AUC, 0.522; 95% confidence interval, 0.474–0.571).
-
Conclusion
- Neither PCT nor CRP helped discriminate bacterial colitis from nonbacterial colitis.
- Acute infectious diarrhea is one of the commonest diseases in the world [1]. Most cases are self-limited and caused by viral pathogens; therefore, routine antibiotics are not recommended [1]. Nevertheless, empirical antibiotic therapy for acute infectious diarrhea is still widely used in clinical practice [2]. Indiscriminate antibiotic use can lead to antibiotic resistance, allergic reactions, drug-related toxicities, Clostridium difficile infection, and increased medical costs [3,4]. Therefore, it is necessary to quickly identify whether a bacterial infection is present in the early stages of acute bacterial colitis. To date, treatment strategies for patients with suspected infectious diarrhea are determined mainly by correctly classifying the severity of the clinical features of the patients; however, there is no objective biochemical indicator for distinguishing bacterial colitis from nonbacterial colitis [1].
- Stool-based tests such as bacterial culture, microscopy, and stool antigen tests are conventional diagnostic approaches for identifying enteric pathogens in patients with bacterial colitis [1]. However, these tests are time-consuming, have inadequate sensitivity, and require specialized equipment for analysis [1,5]. C-reactive protein (CRP) and procalcitonin (PCT) are representative serological markers for various inflammatory conditions and sepsis [6]. CRP is an acute-phase reactant used to diagnose and follow up on diverse bacterial infections [6]. PCT is a calcitonin precursor made of 116 amino acids [7]. PCT is rarely expressed under normal conditions, but it is activated in response to bacterial infection and mediated by endotoxins or interleukins (ILs) and tumor necrosis factor-α [7]. Regarding speed, simplicity, and expense, if bacterial colitis can be differentiated by serologic biomarkers such as PCT and CRP, it will be beneficial in making clinical treatment decisions.
- To date, there is insufficient data on the clinical feasibility of PCT and CRP in diagnosing acute bacterial colitis. Herein, we evaluated the value of serum PCT and CRP in differentiating between bacterial and nonbacterial colitis.
Introduction
- Ethical statements: This study was approved by the Institutional Review Board (IRB) of Keimyung University Dongsan Medical Center (IRB No: 2021-07-075). The requirement for informed consent was waived owing to the study’s retrospective design.
- 1. Patients and study design
- Between November 2014 and April 2021, we retrospectively reviewed the medical records of adult patients who experienced watery diarrhea three or more times a day with abdominal symptoms such as nausea, vomiting, and abdominal pain within 14 days of visiting the hospital. Only patients with serological and stool pathogen polymerase chain reaction (PCR) test results were finally included in this study within 24 hours of visiting the hospital. Based on PCR (CFX96 Real-time System, Bio-Rad, Hercules, CA, USA) results, patients were divided into bacterial and nonbacterial colitis groups. Additionally, results of laboratory tests, including serum PCT, CRP, white blood cell (WBC) count, platelet, aspartate transaminase, alanine aminotransferase, albumin, blood urea nitrogen, creatinine, and clinical parameters (such as fever and admission to the intensive care unit [ICU]), comorbidities and presence of bacteremia were collected. These variables were compared between the two groups.
- 2. Laboratory tests
- Serum CRP levels were measured using the nephelometric method with Roche Cobas C 702 (Roche, Tokyo, Japan). Serum PCT levels were measured using the chemiluminescence method in a Cobas E 801 analyzer (Roche, Japan). Multiplex stool PCR (SEEAMP, CFX96 Real-time System, Bio-Rad) was used for the stool pathogen PCR test, which could reveal Salmonella, Shigella, Vibrio, Campylobacter, Escherichia coli O157:H7, Aeromonas, C. difficile toxin, Clostridium perfringens, Yersinia enterocolitica, and verotoxin-producing E. coli, as causative agents.
- 3. Definition of bacterial colitis
- A patient with the clinical features of colitis and detected bacterial pathogens with a stool pathogen PCR test was considered as having bacterial colitis [8]. Patients in whom bacterial pathogens were not detected with the multiplex stool PCR test were considered as having nonbacterial colitis. The clinical manifestations of colitis include fever (body temperature of >37.8°C), abdominal pain, nausea, vomiting, and diarrhea.
- 4. Statistical analysis
- Statistical analysis was performed using IBM SPSS ver. 24.0 (IBM Corp., Armonk, NY, USA). Categorical variables are expressed as the number and percentage of the participants, and continuous variables are expressed as mean and standard deviation. We used the chi-square analysis for categorical variables and the t-tests for continuous variables. A p-value less than 0.05 was considered statistically significant. Receiver operating characteristic (ROC) curve analysis was used to assess the performance of PCT and CRP in differentiating between bacterial and nonbacterial colitis. The cutoff values were confirmed using the Youden index.
Methods
- In total, 638 patients were included in this study: 186 in the bacterial colitis group and 452 in the nonbacterial colitis group. Table 1 presents the patients’ baseline characteristics. The proportion of patients aged ≥65 years was significantly higher in the bacterial colitis group than in the nonbacterial colitis group (60.8% vs. 50.2%, p=0.015). Patients with chemotherapy were significantly higher in the bacterial colitis group than in the nonbacterial colitis group (16.1% vs. 8.4%, p=0.007). Serum CRP level was significantly higher in the bacterial colitis group than in the nonbacterial colitis group (mean±standard deviation, 11.98±9.75 vs. 10.11±9.34 mg/dL, p=0.023). The mean serum PCT level was 8.46 ng/mL in the bacterial colitis group and 7.98 ng/mL in the nonbacterial colitis group; there was no significant difference between the groups. Other laboratory values and clinical parameters, including WBC count, platelet count, creatinine level, fever, ICU admission, use of immunosuppressant, and presence of bacteremia, were not significantly different between the two groups. The causative pathogens detected by PCR testing are presented in Table 2. In the bacterial colitis group, C. perfringens was the commonest pathogen (n=70, 37.6%), followed by C. difficile toxin B (n=65, 34.9%), Campylobacter spp. (n=42, 22.6%), and Salmonella spp. (n=11, 5.9%).
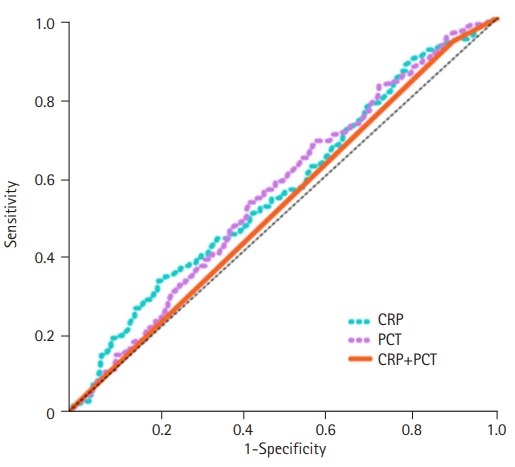
- Findings of the ROC curve analysis of PCT and CRP in differentiating between bacterial and nonbacterial colitis are shown in Table 3 and Fig. 1. The area under the curve (AUC) of PCT for diagnosing bacterial colitis was 0.557 (95% confidence interval [CI], 0.509–0.605). At a cutoff of 0.52 ng/mL, the sensitivity and specificity of PCT were 54.8% and 54.6%, respectively. The AUC of CRP was 0.561 (95% CI, 0.512–0.610). At a cutoff level of 8.80 ng/mL, the sensitivity and specificity of CRP were 52.2% and 54.2%, respectively. Combining PCT and CRP did not improve the diagnostic performance beyond that of PCT or CRP alone (AUC, 0.522; 95% CI, 0.474–0.571).
Results
- Our study demonstrated that serum PCT and CRP levels were not potential markers for the early distinction between bacterial and nonbacterial colitis. The combination of PCT and CRP did not show a better diagnostic performance in discriminating bacterial colitis from nonbacterial colitis than PCR or CRP alone.
- In Korea, among nosocomial infections, the rate of methicillin-resistant Staphylococcus aureus reached 80%, and the vancomycin-resistant enterococci rate of Enterococcus faecium reached 24% [9]. If the bacterial infection can be predicted early, patient treatment can be determined in advance to prevent unnecessary antibiotics. This will ultimately help address issues such as the overuse of antibiotics, rising healthcare costs, and antibiotic resistance. For this reason, several biomarkers are being actively studied as tools to distinguish bacterial infections (e.g., leucocyte count, erythrocyte sedimentation rate, CRP, soluble triggering receptor expressed on myeloid cells 1, serum PCT, IL-6, IL-8, IL-27, etc.) [10].
- An ideal biomarker should not be only highly sensitive and specific but also easy to use, fast, and inexpensive [11,12]. CRP, the most commonly used serologic biomarker for infection, rises 12 to 24 hours after infection and reaches its maximum level after 24 hours. On the other hand, PCT is immediately detectable within 3 to 4 hours after the infection and reaches its maximum level 6 to 12 hours later [13]. When infection is controlled, PCT levels decrease rapidly. In addition, PCT seems to be useful for diagnosing bacterial infection because its level is reduced by IL-γ, a viral infection mediator [14]. Due to the immediate response of PCT to bacterial infection, PCT is thought to be a promising biomarker for early diagnosis of bacterial infection, monitoring of response to antibiotics, or determination of the need to change antibiotics [13]. CRP can also increase during exacerbation of viral infection or autoimmune disease [15], but PCT is less affected by conditions, such as neutropenia and reduced immunity, than CRP [14].
- The diagnostic value of PCT is a focus of research as PCT is reportedly useful for early diagnosis and monitoring of various diseases, especially bacterial infections. In a systematic review and meta-analysis, PCT showed better diagnostic accuracy than CRP in hospitalized patients with suspected bacterial infections [6]. PCT is reportedly a potential marker for septic shock in acute cholangitis [16]. Additionally, PCT plays an important role in differentiating between edematous pancreatitis and necrotizing pancreatitis [17], diagnosing respiratory distress syndrome [18], and monitoring infections in transplant patients [19].
- Although the feasibility of PCT has been studied in various diseases, only a few studies have addressed its role as a diagnostic biomarker for bacterial colitis. One previous study analyzed the discriminative value of PCT in differentiating between inflammatory and noninflammatory diarrhea [20]. In that study, serum PCT had a significant predictive value (odds ratio [OR], 1.321; AUC, 0.797) and a better predictive value than CRP (OR, 1.145; AUC, 0.697) [20]. Contrarily in our study, neither PCT nor CRP helped discriminate between bacterial and nonbacterial colitis. Unlike in our study, in a previous study, inflammatory colitis was diagnosed using colonoscopy and imaging, and no tests for microbial pathogens were used. The results may differ because the studies used different definitions of bacterial colitis; however, our study had a larger sample size and used multiple PCR testing for diagnosis, which is more sensitive than culture [1,21]. In a study investigating whether PCT could differentiate between infectious gastroenteritis and inflammatory bowel disease (IBD), PCT and CRP appeared to be good diagnostic markers for gastroenteritis. Still, there was no significant difference in IBD monitoring using PCT. However, similar to our study, studies have shown that PCT is not an appropriate diagnostic tool for bacterial infection. In another study, the feasibility of PCT levels in discriminating Salmonella infections was assessed: PCT had a low diagnostic value [22].
- In this study, C. perfringens and C. difficile accounted for most colitis-associated bacterial infections. Contrarily, E. coli is known to be the commonest cause of infectious diarrhea in Korea, followed by S. aureus, Salmonella, Vibrio parahaemolyticus, C. perfringens, Bacillus cereus, Campylobacter jejuni, and Shigella [8]. Since this study targeted patients who visited a general tertiary hospital, the distribution of the causative bacteria may differ slightly from that of the general population.
- This study has some limitations. First, there may have been a selection bias, given the study’s retrospective design. Second, PCT reaches peak levels 6 to 24 hours after infection; however, in this retrospective study, blood sampling may have been performed when PCT levels were not sufficiently elevated. Third, PCT levels could have been higher in patients with cancer or immune diseases, but the patients’ comorbidities and medications were not included in the analyses in this study. Fourth, because the fecal PCR test is not a test tool that can accurately diagnose all pathogens, even in patients with bacterial colitis, bacterial pathogens may not be detected by fecal PCR testing. In particular, although C. difficile-associated diarrhea (CDAD) has pathophysiological differences from other bacterial colitis, mild CDAD patients may have been included in the nonbacterial colitis group in our study. Nevertheless, the advantage of this study is that it identified the feasibility of PCT, CRP, and stool PCR testing in a relatively large number of patients. This is the first study to investigate the feasibility of PCT and CRP in differentiating between bacterial and nonbacterial colitis based on the stool PCR test.
- In conclusion, our study showed that neither serum PCT nor CRP levels helped differentiate between bacterial and nonbacterial colitis. Although serum PCT or CRP may be helpful in the clinical judgment of bacterial colitis when considered in conjunction with history, physical examination, and other laboratory values, caution is warranted in differentiating between bacterial and nonbacterial colitis using PCT or CRP alone. Larger prospective studies will help validate these results.
Discussion
-
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
-
Funding
None.
-
Author contributions
Conceptualization, Formal analysis: JYL, YJL, JWL, JSK, JYL, BKJ, WJC, KBC, JSH; Data curation, Project administration, Visualization, Software: JYL, YJL; Investigation, Resources: JYL; Methodology: YJL; Supervision: YJL, JWL, JSK, JYL, BKJ; Writing-original draft: JYL, SYL; Writing-review & editing: JYL, SYL.
Notes

| Type | Data |
|---|---|
| Clostridium perfringens | 70 (37.6) |
| Clostridium difficile toxin B | 65 (34.9) |
| Campylobacter spp. | 42 (22.6) |
| Salmonella spp. | 11 (5.9) |
| Shigella | 6 (3.2) |
| VTEC | 1 (0.5) |
| Index | AUC (95% CI) | Cutoff | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| PCT | 0.557 (0.509–0.605) | 0.52 | 54.8 | 54.6 |
| CRP | 0.561 (0.512–0.610) | 8.80 | 52.2 | 54.2 |
| PCT+CRP | 0.522 (0.474–0.571) | 0.52, 8.80 | 67.2 | 39.3 |
- 1. Riddle MS, DuPont HL, Connor BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol 2016;111:602–22.ArticlePubMedPDF
- 2. Lee HJ, Park KH, Park DA, Park J, Bang BW, Lee SS, et al. Prescription of antibiotics for adults with acute infectious diarrhea in Korea: a population-based study. Infect Chemother 2019;51:295–304.ArticlePubMedPMCPDF
- 3. Schuetz P, Chiappa V, Briel M, Greenwald JL. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med 2011;171:1322–31.ArticlePubMed
- 4. Kim YJ, Park KH, Park DA, Park J, Bang BW, Lee SS, et al. Guideline for the antibiotic use in acute gastroenteritis. Infect Chemother 2019;51:217–43.ArticlePubMedPMCPDF
- 5. Humphries RM, Linscott AJ. Practical guidance for clinical microbiology laboratories: diagnosis of bacterial gastroenteritis. Clin Microbiol Rev 2015;28:3–31.ArticlePubMedPMCPDF
- 6. Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis 2004;39:206–17.ArticlePubMed
- 7. Becker KL, Nylén ES, White JC, Müller B, Snider RH. Clinical review 167: procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab 2004;89:1512–25.ArticlePubMed
- 8. The Korean Society of Infectious Diseases; Korean Society for Chemotherapy; The Korean Society of Clinical Microbiology. Clinical guideline for the diagnosis and treatment of gastrointestinal infections. Infect Chemother 2010;42:323–61.Article
- 9. Song JH. Current status and future strategies of antimicrobial resistance in Korea. Korean J Med 2009;77:143–51.
- 10. Mohan A, Harikrishna J. Biomarkers for the diagnosis of bacterial infections: in pursuit of the ‘Holy Grail’. Indian J Med Res 2015;141:271–3.ArticlePubMedPMC
- 11. Wang J, Niu R, Jiang L, Wang Y, Shao X, Wu M, et al. The diagnostic values of C-reactive protein and procalcitonin in identifying systemic lupus erythematosus infection and disease activity. Medicine (Baltimore) 2019;98:e16798.ArticlePubMedPMC
- 12. Oh HH, Joo YE. Novel biomarkers for the diagnosis and prognosis of colorectal cancer. Intest Res 2020;18:168–83.ArticlePubMedPMCPDF
- 13. Samsudin I, Vasikaran SD. Clinical utility and measurement of procalcitonin. Clin Biochem Rev 2017;38:59–68.PubMedPMC
- 14. Schuetz P, Christ-Crain M, Müller B. Procalcitonin and other biomarkers to improve assessment and antibiotic stewardship in infections: hope for hype? Swiss Med Wkly 2009;139:318–26.ArticlePubMed
- 15. Mitaka C. Clinical laboratory differentiation of infectious versus non-infectious systemic inflammatory response syndrome. Clin Chim Acta 2005;351:17–29.ArticlePubMed
- 16. Lee YS, Cho KB, Park KS, Lee JY, Lee YJ. Procalcitonin as a decision-supporting marker of urgent biliary decompression in acute cholangitis. Dig Dis Sci 2018;63:2474–9.ArticlePubMedPDF
- 17. Rau B, Steinbach G, Gansauge F, Mayer JM, Grünert A, Beger HG. The potential role of procalcitonin and interleukin 8 in the prediction of infected necrosis in acute pancreatitis. Gut 1997;41:832–40.ArticlePubMedPMC
- 18. Brunkhorst FM, Eberhard OK, Brunkhorst R. Discrimination of infectious and noninfectious causes of early acute respiratory distress syndrome by procalcitonin. Crit Care Med 1999;27:2172–6.ArticlePubMed
- 19. Kuse ER, Langefeld I, Jaeger K, Külpmann WR. Procalcitonin in fever of unknown origin after liver transplantation: a variable to differentiate acute rejection from infection. Crit Care Med 2000;28:555–9.ArticlePubMed
- 20. Shin HJ, Kang SH, Moon HS, Sung JK, Jeong HY, Kim JS, et al. Serum procalcitonin levels can be used to differentiate between inflammatory and non-inflammatory diarrhea in acute infectious diarrhea. Medicine (Baltimore) 2018;97:e11795.ArticlePubMedPMC
- 21. Kim SH, Kim YS, Kim SH, Yoon WE, Myung HJ, Moon JS, et al. Usefulness of stool multiplex polymerase chain reaction assays in patients with acute diarrhea. Korean J Gastroenterol 2022;79:118–25.ArticlePubMed
- 22. Mishra V, Sorabjee J. Procalcitonin levels in salmonella infection. Indian J Crit Care Med 2015;19:471–3.ArticlePubMedPMC
References
Figure & Data
References
Citations


 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine PubReader
PubReader ePub Link
ePub Link Cite
Cite