PubMed Central, CAS, DOAJ, KCI

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Volume 40(4); 2023 > Article
-
Case report
Interleukin-6-producing paraganglioma as a rare cause of systemic inflammatory response syndrome: a case report -
Yin Young Lee1
 , Seung Min Chung2
, Seung Min Chung2
-
Journal of Yeungnam Medical Science 2023;40(4):435-441.
DOI: https://doi.org/10.12701/jyms.2022.00766
Published online: March 7, 2023
1Division of Endocrinology and Metabolism, Department of Internal Medicine, Daegu Veterans Hospital, Daegu, Korea
2Division of Endocrinology and Metabolism, Department of Internal Medicine, Yeungnam University College of Medicine, Daegu, Korea
- Corresponding author: Seung Min Chung, MD, PhD Division of Endocrinology and Metabolism, Department of Internal Medicine, Yeungnam University College of Medicine, 170 Hyunchung-ro, Nam-gu, Daegu 42415, Korea Tel: +82-53-620-4292 • Fax: +82-53-654-8386 • E-mail: smchung@ynu.ac.kr
Copyright © 2023 Yeungnam University College of Medicine, Yeungnam University Institute of Medical Science
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,740 Views
- 67 Download
Abstract
- Pheochromocytomas and paragangliomas (PPGLs) may secrete hormones or bioactive neuropeptides such as interleukin-6 (IL-6), which can mask the clinical manifestations of catecholamine hypersecretion. We report the case of a patient with delayed diagnosis of paraganglioma due to the development of IL-6-mediated systemic inflammatory response syndrome (SIRS). A 58-year-old woman presented with dyspnea and flank pain accompanied by SIRS and acute cardiac, kidney, and liver injuries. A left paravertebral mass was incidentally observed on abdominal computed tomography (CT). Biochemical tests revealed increased 24-hour urinary metanephrine (2.12 mg/day), plasma norepinephrine (1,588 pg/mL), plasma normetanephrine (2.27 nmol/L), and IL-6 (16.5 pg/mL) levels. 18F-fluorodeoxyglucose (FDG) positron emission tomography/CT showed increased uptake of FDG in the left paravertebral mass without metastases. The patient was finally diagnosed with functional paraganglioma crisis. The precipitating factor was unclear, but phendimetrazine tartrate, a norepinephrine-dopamine release drug that the patient regularly took, might have stimulated the paraganglioma. The patient’s body temperature and blood pressure were well controlled after alpha-blocker administration, and the retroperitoneal mass was surgically resected successfully. After surgery, the patient’s inflammatory, cardiac, renal, and hepatic biomarkers and catecholamine levels improved. In conclusion, our report emphasizes the importance of IL-6-producing PPGLs in the differential diagnosis of SIRS.
- Pheochromocytomas and paragangliomas (PPGLs) are rare neuroendocrine tumors arising from chromaffin cells of the adrenal medulla and autonomic neural ganglia that may secrete catecholamines [1]. PPGL usually presents with various symptoms such as paroxysmal hypertension, headache, palpitations, diaphoresis, and tachycardia due to excessive catecholamine secretion. However, some PPGLs may be associated with the secretion of other hormones, such as adrenocorticotropic hormone, cortisol, or bioactive neuropeptides, resulting in unusual symptoms that complicate diagnosis [2]. One such peptide is interleukin-6 (IL-6), a multifunctional cytokine with pivotal roles in immune and inflammatory responses [3]. IL-6 stimulates the differentiation of B lymphocytes, activation of T lymphocytes, and regulates the synthesis of acute-phase proteins, such as C-reactive protein (CRP) and fibrinogen [2]. In addition, overproduction of IL-6 induces systemic inflammatory response syndrome (SIRS) in patients with PPGL [4]. In this report, we describe the case of a 58-year-old woman with a paraganglioma, who presented with SIRS and elevated plasma IL-6 levels.
Introduction
- Ethical statements: This study was exempted from review by the Institutional Review Board (IRB) of Yeungnam University Hospital (IRB No: 2022-09-052), which waived the requirement of informed consent from patients.
- In 2021, a 58-year-old woman presented to the emergency department with dyspnea and bilateral flank pain. She presented with pallor and clamminess and had a blood pressure of 170/120 mmHg, heart rate of 120 beats/min, body temperature of 38.2°C, respiration rate of 30 breaths/min, oxygen saturation of 89% on room air, and a body mass index of 25.1 kg/m2 (height, 148 cm; weight, 55 kg). She had no medical history or significant family history but had been taking phendimetrazine tartrate for weight loss for 5 years. On physical examination, crackles were heard in the right lung field upon auscultation and the abdomen was soft without tenderness.
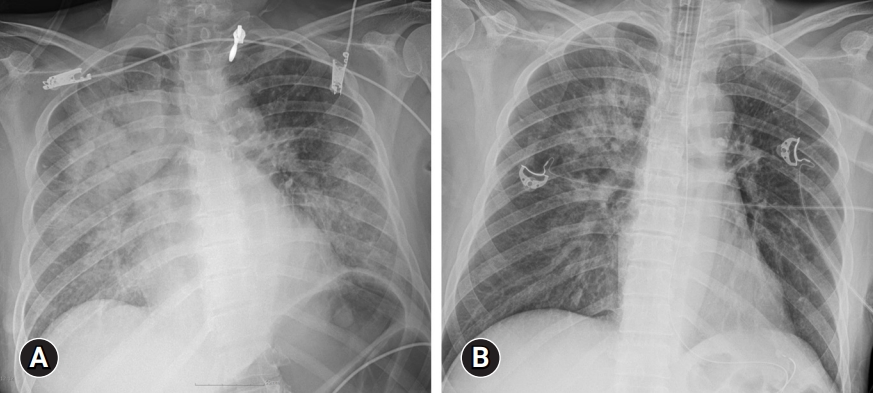
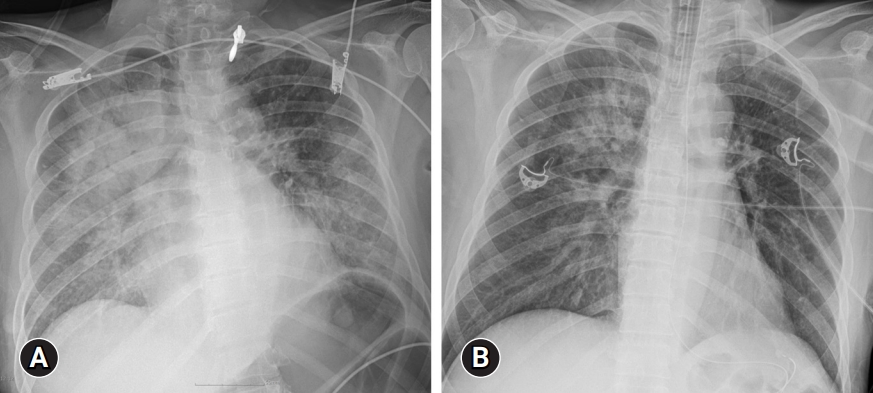
- Laboratory findings revealed elevated levels of inflammatory markers, including a CRP of 29.5 mg/dL and an erythrocyte sedimentation rate (ESR) of 120 mm/hr. Furthermore, leukocytosis (14.82×103/μL) and normocytic anemia (hemoglobin, 9.9 g/dL; mean corpuscular volume, 90.7 fL) were detected, and her platelet count was normal. Suspicious acute kidney injury (blood urea nitrogen, 25 mg/dL; creatinine, 1.66 mg/dL) and liver injury (aspartate aminotransferase, 179 IU/L; alanine aminotransferase, 106 IU/L) were observed (Table 1). Arterial blood gas analysis revealed mixed metabolic-respiratory acidosis with markedly elevated lactate levels (20 mmol/L). Chest radiography revealed bilateral diffuse infiltrations (Fig. 1A). Chest and abdominal computed tomography (CT) revealed a round mass lesion measuring 3.8 cm in length with heterogeneous contrast enhancement in the left paravertebral area (Fig. 2A, 2B).
- Considering the sepsis of unknown origin and acute respiratory distress syndrome, the patient was sedated, intubated, and admitted to the intensive care unit. Within a 24-hour period of starting broad-spectrum antibiotics (vancomycin, meropenem, azithromycin) and mechanical ventilation, chest radiography showed rapid resolution of the lung infiltrations (Fig. 1B). However, the high fever and uncontrolled hypertension persisted in the patient. In addition, the patient’s troponin-I (0.841 ng/mL), creatinine kinase (5.3 ng/mL), and N-terminal prohormone of brain natriuretic peptide (NT-proBNP; 35,000 pg/mL) levels were elevated (Table 1). A bedside transthoracic echocardiogram showed hypokinetic mid inferior and apical segments and a reduced left ventricular ejection fraction of 38%; however, the echocardiogram could not explain the patient’s fever and paroxysmal hypertension.
- The patient was referred to an endocrinologist for an incidentally discovered extra-adrenal mass and clinical signs and symptoms suggesting the likelihood of PPGL (such as pallor, palpitations, and tachycardia). In urinary and plasma catecholamine and metanephrine analysis, the 24-hour urinary metanephrine (2.12 mg/day; range, 0–0.71 mg/day), plasma norepinephrine (1,588 pg/mL; range, 70–150 pg/mL), and normetanephrine (2.27 nmol/L; range, <0.90 nmol/L) were elevated, whereas plasma epinephrine (18 pg/mL; range, 0–110 pg/mL), and metanephrine (0.04 nmol/L; range, <0.50 nmol/L) were normal (Table 1). In addition, the plasma IL-6 concentration appeared to be elevated (16.5 pg/mL; range, 0–7.0 pg/mL). Since somatostatin receptor positron emission tomography (PET)/CT was not available in our hospital, 18F-fluorodeoxyglucose PET/CT was the second choice, which showed increased uptake in the left paravertebral mass, without metastases (Fig. 2C).
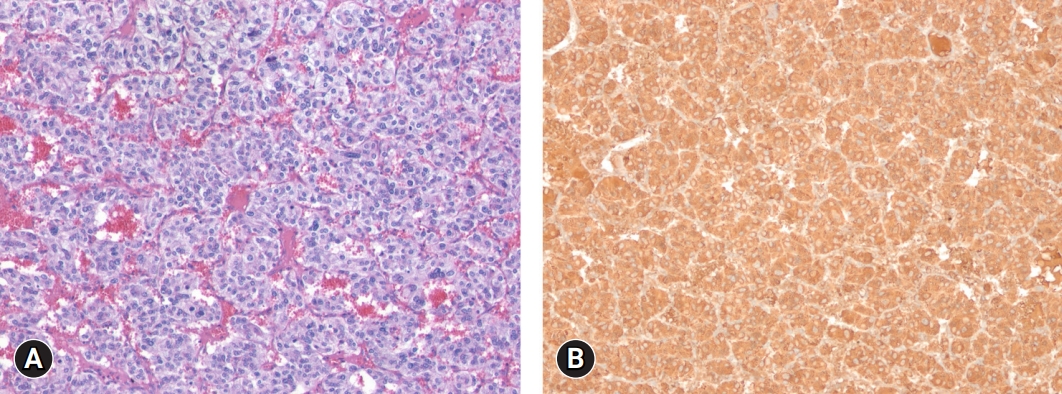
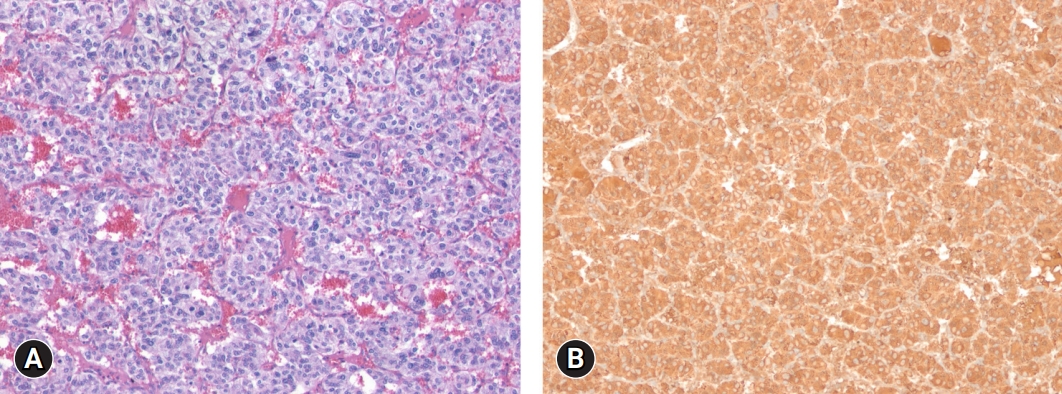
- A diagnosis of functional paraganglioma was established by integrating the clinical, laboratory, and imaging data. The combination of a newly diagnosed paraganglioma with persistent fever and elevated levels of inflammatory markers raised suspicion of IL-6 production. Although the family history was negative, a succinate dehydrogenase complex iron sulfur subunit B (SDHB) mutation [c.541-3C>R, p(?) heterozygous], a variant of uncertain significance, was found. After initiating preoperative alpha-blockers (doxazosin), the body temperature normalized to 36.5°C, and blood pressure was well controlled. The patient underwent open excision of the retroperitoneal mass after the administration of doxazosin for 17 days. The excised tumor was 4.3×3.5×3.7 cm3 and was well encapsulated. Histological examination of the tumor revealed a prominent cell-nesting pattern (zellballen). The cells had round to ovoid nuclei and abundant basophilic granular cytoplasm. Immunohistochemistry for chromogranin A showed diffuse positive staining of the tumor cells (Fig. 3). Postoperatively, doxazosin was discontinued and no postoperative complications were observed. Four days after surgery, the patient’s inflammatory, cardiac, renal, and hepatic biomarkers improved, and 24-hour urinary metanephrine levels decreased to within normal range (0.25 mg/day) (Table 1). Because the patient was diagnosed with diabetes and wished to continue weight-loss medication, phendimetrazine tartrate was replaced with a glucagon-like peptide-1 receptor agonist. Follow-up abdominal CT performed 13 months after tumor removal revealed no evidence of recurrence or distant metastases. The patient was asymptomatic during the follow-up visits.
Case
- Here, we present a case of paraganglioma crisis that presented with fever. The precipitating factor was unclear, but chronic intake of phendimetrazine tartrate, a norepinephrine-dopamine-releasing agent, might have stimulated the paraganglioma [1]. This case report presents important clinical considerations. Although pyrexia is not a typical manifestation of paraganglioma, the patient’s clinical course was observed in the form of SIRS, which delayed accurate diagnosis. To date, 45 cases of IL-6-producing PPGLs have been reported [5]. However, the actual incidence might be higher given that PPGLs may often be overlooked owing to a lack of awareness of the diagnosis or masked clinical manifestations caused by catecholamine hypersecretion [6].
- IL-6 is a pivotal cytokine produced in response to infections and tissue injuries that contributes to host defense through the stimulation of acute-phase responses, hematopoiesis, and immune responses [2]. IL-6 acts as an endogenous pyrogen, which may result in a rare clinical manifestation of PPGL with a fever of unknown origin. The exact origin of excessive IL-6 secretion in patients with PPGLs has not been elucidated. Cheng et al. [7] reported that patients with high body temperature and IL-6 levels had significantly higher IL-6 protein expression (measured immunohistochemically in pheochromocytoma tissue) than patients with normal body temperature and low IL-6 levels. Similarly, other studies have shown increased IL-6 expression in resected tumors in PPGLs patients with increased plasma IL-6 and inflammatory marker levels [8,9]. Another study has suggested that tumors secrete IL-6 because of high circulating norepinephrine levels [10]. However, Cheng et al. [7] reported cases of patients with pheochromocytomas and IL-6 overproduction even without norepinephrine excess. Therefore, IL-6 is estimated to be synthesized and secreted by PPGL neoplastic cells [5]. Unfortunately, we did not perform IL-6 immunohistochemical testing of the patient’s tumor.
- Laboratory abnormalities in this patient, including leukocytosis, anemia, and upregulated levels of inflammatory markers such as CRP and ESR, can be attributed to elevated IL-6 levels. IL-6 inhibits iron supply and the proliferation of erythroid progenitor cells, resulting in anemia related to chronic inflammation [8]. In addition, IL-6 may be involved in the activation of polyclonal B cells, differentiation of B cells into plasma cells, and stimulation of megakaryocyte development, resulting in other hematological abnormalities, such as leukocytosis [9]. Some studies have indicated that laboratory values, including IL-6 levels, are normalized after tumor resection [6]. This supports the hypothesis that the abnormal laboratory findings for this patient resulted from IL-6 overproduction, as the patient’s laboratory values and clinical symptoms improved after tumor resection. The patient had no family history, and although she had been using sympathomimetics for 5 years, she exhibited no symptoms. The patient was diagnosed with SDHB mutation-associated paraganglioma. Although mutation carriers are often found in the absence of family history [11], genetic testing is recommended for all patients diagnosed with PPGLs, because they have the highest rate of heritability among all tumors. The Korean Endocrine Society has proposed a basic panel of 10 genes (FH, MAX, NF1, RET, SDHA, SDHB, SDHC, SDHD, TMEM127, and HL) and an extended panel of 15 genes (basic panel genes plus EGLN1/PHD2, EPAS1, KIF1B, MET, and SDHAF2) for targeted next-generation sequencing (NGS)-based diagnostic testing for hereditary PPGLs [12]. In this patient, as the diagnosis was a nonmetastatic, extra-adrenal, and noradrenergic PPGL, pseudohypoxia-related cluster 1 gene panel examination (1A - Krebs cycle-related: SDHx (SDHA, B, C, D, F2), FH, and MDH2; and 1B - VHL/EPAS1-related: VHL, EPAS1) may be considered [13]. Although our study only confirmed the SDHB mutation by direct sequencing, NGS should be performed in future studies. SDHB mutations are the most common gene mutations in PPGLs, and these mutations are associated with more aggressive tumors, younger age of onset, and higher metastasis rates [14,15]. SDHB mutations tend to be related to abdominal or thoracic extra-adrenal paragangliomas and usually exhibit noradrenergic or dopaminergic phenotypes [11,16].
- Catecholamine tests are affected by various factors (medications, inappropriate sampling, measurement methods, physiological stress, and diet); therefore, the possibility of false-positive results should be considered when interpreting the results. In this study, the patient’s plasma normetanephrine level (2.27 nmol/L, equivalent to 654.895 ng/L) largely increased to more than twice the normal upper limit, indicating a high possibility of a PPGL and lower probability of a false positive [17]. The 24-hour urine metanephrine concentrations were also found to be elevated. Since the patient was under stress and we continued to administer acetaminophen for fever control, there is a possibility that these factors may have resulted in the positive 24-hour urine samples.
- Although surgical resection of the tumor is the only curative treatment for IL-6-producing PPGLs, alpha-blockers and nonsteroidal anti-inflammatory drugs have been suggested as pharmacological alternatives to improve the clinical manifestations of IL-6 overproduction [9,18,19]. An investigation of the anti-inflammatory effects of the α1-adrenergic receptor antagonist doxazosin revealed that it inhibits the production of tumor necrosis factor α and monocyte chemoattractant protein-1 in mice; thus, it is effective in patients with IL-6-secreting PPGLs [20]. Some case reports have shown resolution of pyrexia and decreased levels of IL-6 after administration of an alpha-blocker, consistent with the developments in our patient [4,10].
- In conclusion, we report the case of a patient with increased IL-6 levels and inflammatory markers in the presence of a paraganglioma. Overproduction of IL-6 appeared to be the primary mediator in developing SIRS, in addition to inducing marked increases in the levels of inflammatory markers. Therefore, evaluation of IL-6 production is recommended in cases of paraganglioma presenting with SIRS.
Discussion
-
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
-
Funding
None.
-
Author contributions
Conceptualization, Formal analysis: YYL, SMC. Data curation, Investigation: YYL. Supervision: SMC. Writing-original draft: YYL. Writing-review & editing: YYL, SMC.
Notes

- 1. Whitelaw BC, Prague JK, Mustafa OG, Schulte KM, Hopkins PA, Gilbert JA, et al. Phaeochromocytoma [corrected] crisis. Clin Endocrinol (Oxf) 2014;80:13–22.ArticlePubMed
- 2. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 2014;6:a016295.ArticlePubMedPMC
- 3. Akira S, Hirano T, Taga T, Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). FASEB J 1990;4:2860–7.ArticlePubMedPDF
- 4. Carvalho Cunha N, Gomes L, Saraiva J, Paiva I. Interleukin-6 producing pheochromocytoma: a rare cause of systemic inflammatory response syndrome. Case Rep Endocrinol 2019;2019:7906272.ArticlePubMedPMC
- 5. Meijs AC, Schroijen MA, Snel M, Corssmit EP. Interleukin-6 producing pheochromocytoma/paraganglioma: case series from a tertiary referral centre for pheochromocytomas and paragangliomas. J Endocrinol Invest 2021;44:2253–9.ArticlePubMedPMCPDF
- 6. Angelousi A, Peppa M, Chrisoulidou A, Alexandraki K, Berthon A, Faucz FR, et al. Malignant pheochromocytomas/paragangliomas and ectopic hormonal secretion: a case series and review of the literature. Cancers (Basel) 2019;11:724.ArticlePubMedPMC
- 7. Cheng X, Zhang M, Xiao Y, Li H, Zhang Y, Ji Z. Interleukin-6-producing pheochromocytoma as a new reason for fever of unknown origin: a retrospective study. Endocr Pract 2018;24:507–11.ArticlePubMed
- 8. Minetto M, Dovio A, Ventura M, Cappia S, Daffara F, Terzolo M, et al. Interleukin-6 producing pheochromocytoma presenting with acute inflammatory syndrome. J Endocrinol Invest 2003;26:453–7.ArticlePubMedPDF
- 9. Kang JM, Lee WJ, Kim WB, Kim TY, Koh JM, Hong SJ, et al. Systemic inflammatory syndrome and hepatic inflammatory cell infiltration caused by an interleukin-6 producing pheochromocytoma. Endocr J 2005;52:193–8.ArticlePubMed
- 10. Yarman S, Soyluk O, Altunoglu E, Tanakol R. Interleukin-6-producing pheochromocytoma presenting with fever of unknown origin. Clinics (Sao Paulo) 2011;66:1843–5.ArticlePubMedPMC
- 11. Martucci VL, Pacak K. Pheochromocytoma and paraganglioma: diagnosis, genetics, management, and treatment. Curr Probl Cancer 2014;38:7–41.ArticlePubMedPMC
- 12. Ku EJ, Kim KJ, Kim JH, Kim MK, Ahn CH, Lee KA, et al. Diagnosis for pheochromocytoma and paraganglioma: a joint position statement of the Korean Pheochromocytoma and Paraganglioma Task Force. Endocrinol Metab (Seoul) 2021;36:322–38.ArticlePubMedPMCPDF
- 13. Nölting S, Bechmann N, Taieb D, Beuschlein F, Fassnacht M, Kroiss M, et al. Personalized management of pheochromocytoma and paraganglioma. Endocr Rev 2022;43:199–239.ArticlePubMedPMCPDF
- 14. Gimenez-Roqueplo AP, Dahia PL, Robledo M. An update on the genetics of paraganglioma, pheochromocytoma, and associated hereditary syndromes. Horm Metab Res 2012;44:328–33.ArticlePubMed
- 15. van Hulsteijn LT, Dekkers OM, Hes FJ, Smit JW, Corssmit EP. Risk of malignant paraganglioma in SDHB-mutation and SDHD-mutation carriers: a systematic review and meta-analysis. J Med Genet 2012;49:768–76.ArticlePubMed
- 16. Benn DE, Gimenez-Roqueplo AP, Reilly JR, Bertherat J, Burgess J, Byth K, et al. Clinical presentation and penetrance of pheochromocytoma/paraganglioma syndromes. J Clin Endocrinol Metab 2006;91:827–36.ArticlePubMed
- 17. Boyd J, Leung AA, Sadrzadeh HS, Pamporaki C, Pacak K, Deutschbein T, et al. A high rate of modestly elevated plasma normetanephrine in a population referred for suspected PPGL when measured in a seated position. Eur J Endocrinol 2019;181:301–9.ArticlePubMedPMC
- 18. Shimizu C, Kubo M, Takano K, Takano A, Kijima H, Saji H, et al. Interleukin-6 (IL-6) producing phaeochromocytoma: direct IL-6 suppression by non-steroidal anti-inflammatory drugs. Clin Endocrinol (Oxf) 2001;54:405–10.ArticlePubMedPDF
- 19. Fiebich BL, Lieb K, Hüll M, Berger M, Bauer J. Effects of NSAIDs on IL-1 beta-induced IL-6 mRNA and protein synthesis in human astrocytoma cells. Neuroreport 1996;7:1209–13.ArticlePubMed
- 20. Tung D, Ciallella J, Cheung PH, Saha S. Novel anti-inflammatory effects of doxazosin in rodent models of inflammation. Pharmacology 2013;91:29–34.ArticlePubMed
References
Figure & Data
References
Citations


 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine PubReader
PubReader ePub Link
ePub Link Cite
Cite