PubMed Central, CAS, DOAJ, KCI

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Volume 40(4); 2023 > Article
-
Resident fellow section: Clinical vignette
Differential diagnosis of suddenly developed motor weakness in bilateral lower extremities of a 79-year-old male patient -
Seong Yeob Kwak1,*
 , Mathieu Boudier-Revéret2
, Mathieu Boudier-Revéret2 , Min Cheol Chang1
, Min Cheol Chang1
-
Journal of Yeungnam Medical Science 2023;40(4):457-460.
DOI: https://doi.org/10.12701/jyms.2022.00787
Published online: January 9, 2023
1Department of Physical Medicine and Rehabilitation, Yeungnam University College of Medicine, Daegu, Korea
2Department of Physical Medicine and Rehabilitation, Centre Hospitalier de l’Université de Montréal, Montréal, QC, Canada
- Corresponding author: Min Cheol Chang, MD Department of Physical Medicine and Rehabilitation, Yeungnam University College of Medicine, 170 Hyeonchung-ro, Nam-gu, Daegu 42415, Korea Tel: +82-53-620-4682 • E-mail: wheel633@gmail.com
- *Seong Yeob Kwak is currently in training.
Copyright © 2023 Yeungnam University College of Medicine, Yeungnam University Institute of Medical Science
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,319 Views
- 53 Download
- A 79-year-old man visited the outpatient clinic of a university hospital with bilateral lower extremity weakness, which suddenly developed two weeks prior to presentation. The patient also experienced constipation and voiding difficulty, which manifested as urinary incontinence. He was taking medications for hypertension and dyslipidemia (bisoprolol fumarate, 2.5 mg, once daily; telmisartan, 40 mg, once daily; and (S)-amlodipine besylate, 2.5 mg, once daily). In addition, the patient had undergone radiofrequency catheter ablation at the cardiology department for atrial fibrillation 1 year prior to this visit and was taking oral anticoagulants (rivaroxaban, 20 mg, once daily) and antiarrhythmics (flecainide acetate, 50 mg, twice daily). The patient was admitted to the Department of Physical Medicine and Rehabilitation for further evaluation and treatment. This study conforms to all RFS-CARE (Resident and Fellow Section-CAse REport) guidelines, and the required information has been reported accordingly (Supplementary Checklist).
Patient information
- Physical examination revealed symmetric motor weakness (manual muscle testing scores on bilateral hip flexors, 3; bilateral knee extensors, 4; bilateral ankle dorsiflexors, 2; bilateral great toe extensors and plantar flexors, 1). Light touch and pin-prick sensations were impaired below the bilateral L2 level. Voluntary anal contraction was impaired, and perianal and deep anal sensations were absent. Motor and sensory functions in the upper extremities were normal, but bilateral knee and ankle jerks were reduced. Furthermore, the Babinski reflex and ankle clonus were not observed.
Clinical findings
- Vital signs and laboratory findings, including complete blood count, electrolytes, transaminase, creatine phosphokinase, C-reactive protein (CRP), erythrocyte sedimentation rate, and urine analysis, were within normal ranges. However, his hemoglobin A1c level was 6.6%, and the patient was diagnosed with diabetes through an oral glucose tolerance test.
- 1. Differential diagnosis
- The following diagnoses were considered.
- Sudden onset weakness in the bilateral lower extremities increases the possibility of a spinal cord infarct. Because the deep tendon reflexes had decreased, the pathological site included the conus medullaris. Notably, a medical history of atrial fibrillation increases the likelihood of developing this disorder [1].
- Although symptoms of spondylotic myelopathy often develop insidiously and gradually aggravate, they can occasionally occur suddenly and rapidly. Spondylotic myelopathy is commonly observed in clinical practice.
- A spinal cord tumor was considered; however, the sudden occurrence of weakness in our patient made this diagnosis less likely.
- The patient was diagnosed with diabetes. In addition, his deep tendon reflexes in the bilateral lower extremities had decreased. Therefore, the possibility of diabetic amyotrophy was considered. However, the patient’s weakness pattern was symmetric, which differs from the asymmetrical neuropathy observed with diabetic amyotrophy [2].
- CRP levels were within the normal range; thus, the possibility of vasculitic neuropathy was low. In addition, sensorimotor deficits from vasculitic neuropathy usually develop asymmetrically [3].
- Guillain-Barré syndrome was considered because the patient presented with symmetrical motor weakness in both lower extremities. The patient may have been in the early stages of Guillain-Barré syndrome, which usually begins in the lower extremities and spreads to the upper extremities. However, 2 weeks after the initial manifestation of the patient's symptoms, his motor weakness was still confined to the lower extremities. Therefore, the possibility of Guillain-Barré syndrome was low.
- 2. Diagnosis and management
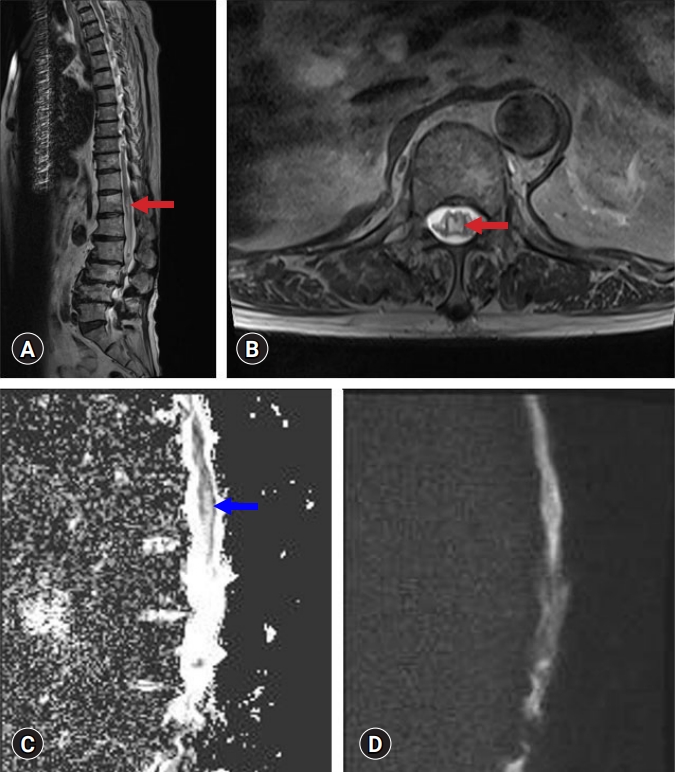
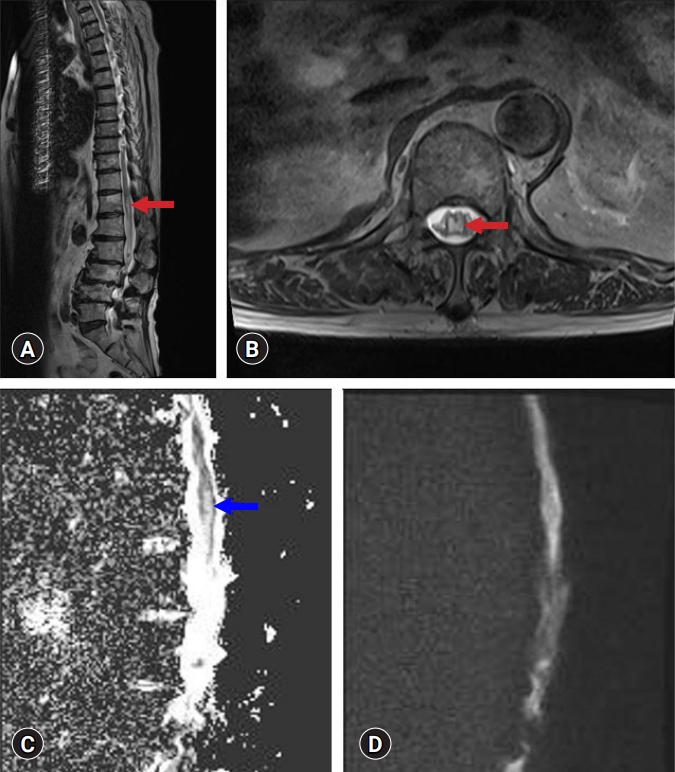
- Whole-spine magnetic resonance imaging (MRI), including diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) mapping, was performed. T2-weighted MRI showed focal swelling with enhanced signal intensity in the central grey matter of the conus medullaris (Fig. 1). Signal hyperintensity at the conus medullaris was found on ADC maps, with no significant restricted diffusion on DWI (Fig. 1). On cervical and thoracic spine MRI, no significant abnormalities were found above the T12 level, and spondylotic myelopathy was excluded. In addition, electrodiagnostic studies, including nerve conduction studies, electromyography, and central motor conduction time, revealed no abnormalities. Therefore, disorders of the peripheral nervous system, such as diabetic amyotrophy, vasculitic neuropathy, and Guillain-Barré syndrome, were excluded.
- The patient was diagnosed with subacute spinal cord infarction [4]. We performed transthoracic echocardiography (TTE) to evaluate cardiac risk factors and computed tomography angiography (CTA) to exclude aortic dissection. No abnormal findings were observed on TTE or CTA. We increased the dose of the oral anticoagulant (rivaroxaban, 20 mg per day to dabigatran etexilate mesylate, 150 mg, twice daily). Exercises for strengthening the lower extremity muscles and improving standing balance and walking ability were conducted (Monday to Friday, 2 hours/day).
Diagnostic assessment
1) Spinal cord infarction
2) Spondylotic myelopathy
3) Spinal cord tumor
4) Diabetic amyotrophy
5) Vasculitic neuropathy
6) Guillain-Barré syndrome
- At the 3-month follow-up after diagnosis, weakness in the right lower extremity of the patient improved (manual muscle testing score on bilateral hip flexors, 4; bilateral knee extensors, 4; bilateral ankle dorsiflexors, 3; bilateral great toe extensors and plantar flexors, 3).
Clinical course
- Our patient was diagnosed with subacute spinal cord infarction. Spinal cord infarction occurs much less frequently than cerebral infarction and accounts for only 1% of all strokes [4]. The most frequent type of spinal cord infarction is anterior spinal artery syndrome, which presents with bilateral weakness, impairment of spinothalamic sensation, and preservation of deep sensations. Rarely, posterior infarcts that spare spinothalamic sensation and involve lemniscal sensation are encountered [5].
- Numerous etiologies have been implicated in spinal cord infarction, including aortic disease, vertebral artery dissection, arterial or cardiac embolism, fibrocartilaginous embolism, hypercoagulable states (e.g., sickle cell disease, antiphospholipid syndrome, and malignancy), decompression sickness, vasculitis, systemic hypotension or global hypoperfusion from cardiac arrest, radicular artery compression from the disc, and trauma [6,7]. The most common identifiable causes are aortic diseases such as aortic dissection or aortic aneurysm [6,8]. CTA of the chest/abdomen should be conducted in patients with thoracic cord infarcts or infarcts of the conus medullaris to evaluate the presence of aortic dissection or aneurysm [7]. Atrial fibrillation is associated with an increased risk of subsequent spinal cord infarction. Therefore, evaluation of potential cardioembolic sources, including echocardiographic evaluation, is needed in patients with unexplained spinal cord infarction [9].
- To date, there are no consensus guidelines regarding the management of spinal cord infarcts. In some previous case studies, anticoagulation and antiplatelet agents were administered in the acute phase of spinal cord infarction in patients who were suspected to have an atherosclerotic etiology. However, the effect of these agents on recovery after spinal cord injury has not been evaluated [7,10]. Similar to other spinal cord injuries, early rehabilitation is important for independence and physical function. It is also necessary to prevent complications from injuries, such as neurogenic bladder and bowel, urinary tract infections, pressure ulcers, orthostatic hypotension, spasticity, deep vein thrombosis, autonomic dysreflexia, and pulmonary problems.
Discussion
- Clinicians should consider the possibility of spinal cord infarction in patients who present with sudden onset weakness in their bilateral extremities, especially in patients with a history of atrial fibrillation or aortic disease.
Conclusion
Supplementary materials
-
Ethical statements
This study was approved by the Institutional Review Board (IRB) of Yeungnam University Hospital (IRB No: 2022-06-031-001). Written informed consent was obtained for the publication of this report.
-
Conflicts of interest
Mathieu Boudier-Revéret has been an editorial board member of Journal of Yeungnam Medical Science (JYMS) since 2021. Min Cheol Chang has been an associate editor of JYMS since 2021. They were not involved in the review process of this manuscript. Otherwise, there is no conflict of interest to declare.
-
Funding
None.
-
Author contributions
Conceptualization, Methodology: all authors; Data curation: SYK, MCC; Formal analysis, Investigation, Resources, Supervision, Validation: MCC; Visualization: SYK; Writing-original draft: all authors; Writing-review & editing: all authors.
Notes
- 1. Cheng MY, Lyu RK, Chang YJ, Chen RS, Huang CC, Wu T, et al. Spinal cord infarction in Chinese patients. Clinical features, risk factors, imaging and prognosis. Cerebrovasc Dis 2008;26:502–8.ArticlePubMedPDF
- 2. Diaz LA, Gupta V. Diabetic amyotrophy [updated 2021 Nov 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 https://www.ncbi.nlm.nih.gov/books/NBK560491/.
- 3. Blaes F. Diagnosis and therapeutic options for peripheral vasculitic neuropathy. Ther Adv Musculoskelet Dis 2015;7:45–55.ArticlePubMedPMCPDF
- 4. Nogueira RG, Ferreira R, Grant PE, Maier SE, Koroshetz WJ, Gonzalez RG, et al. Restricted diffusion in spinal cord infarction demonstrated by magnetic resonance line scan diffusion imaging. Stroke 2012;43:532–5.ArticlePubMed
- 5. Novy J. Spinal cord syndromes. Front Neurol Neurosci 2012;30:195–8.ArticlePubMed
- 6. Nedeltchev K, Loher TJ, Stepper F, Arnold M, Schroth G, Mattle HP, et al. Long-term outcome of acute spinal cord ischemia syndrome. Stroke 2004;35:560–5.ArticlePubMed
- 7. Nasr DM, Rabinstein A. Spinal cord infarcts: risk factors, management, and prognosis. Curr Treat Options Neurol 2017;19:28.ArticlePubMedPDF
- 8. Cheshire WP, Santos CC, Massey EW, Howard JF Jr. Spinal cord infarction: etiology and outcome. Neurology 1996;47:321–30.ArticlePubMed
- 9. Mir S, Pishanidar S, Merkler A. Association between atrial fibrillation and spinal cord infarction (P3.108). Neurology 2017;88(16 Suppl):P3.108.Article
- 10. Zalewski NL, Rabinstein AA, Krecke KN, et al. Characteristics of spontaneous spinal cord infarction and proposed diagnostic criteria. JAMA Neurol 2019;76:56–63.ArticlePubMed
References
Figure & Data
References
Citations


 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine PubReader
PubReader ePub Link
ePub Link Cite
Cite