PubMed Central, CAS, DOAJ, KCI

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Volume 39(4); 2022 > Article
-
Review article
Optogenetic neuromodulation with gamma oscillation as a new strategy for Alzheimer disease: a narrative review -
Haneol Ko1
 , Sang-Pil Yoon2
, Sang-Pil Yoon2
-
Journal of Yeungnam Medical Science 2022;39(4):269-277.
DOI: https://doi.org/10.12701/jyms.2021.01683
Published online: February 14, 2022
1Medical Course, Jeju National University School of Medicine, Jeju, Korea
2Department of Anatomy, Jeju National University College of Medicine, Jeju, Korea
- Corresponding author: Sang-Pil Yoon, MD, PhD Department of Anatomy, Jeju National University College of Medicine, 102 Jejudaehak-ro, Jeju 63243, Korea Tel: +82-64-754-3823 • Fax: +82-64-725-2593 • E-mail: spyoon@jejunu.ac.kr
Copyright © 2022 Yeungnam University College of Medicine, Yeungnam University Institute of Medical Science
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Abstract
- Introduction
- Optogenetic technique as a new neuromodulatory method
- Gamma oscillation entrainment and Alzheimer disease
- Optogenetic neuromodulation and Alzheimer disease
- Optogenetics-induced gamma oscillations and Alzheimer disease
- Limitations and prospects of optogenetics
- Conclusion
- Notes
- References
Abstract
- The amyloid hypothesis has been considered a major explanation of the pathogenesis of Alzheimer disease. However, failure of phase III clinical trials with anti-amyloid-beta monoclonal antibodies reveals the need for other therapeutic approaches to treat Alzheimer disease. Compared to its relatively short history, optogenetics has developed considerably. The expression of microbial opsins in cells using genetic engineering allows specific control of cell signals or molecules. The application of optogenetics to Alzheimer disease research or clinical approaches is increasing. When applied with gamma entrainment, optogenetic neuromodulation can improve Alzheimer disease symptoms. Although safety problems exist with optogenetics such as the use of viral vectors, this technique has great potential for use in Alzheimer disease. In this paper, we review the historical applications of optogenetic neuromodulation with gamma entrainment to investigate the mechanisms involved in Alzheimer disease and potential therapeutic strategies.
- Alzheimer disease (AD), age-dependent dementia characterized by irreversible and progressive loss of memory and cognition, shows an approximately 11.3% prevalence in patients aged 65 years and older in the United States [1]. The prevalence of dementia was reported to be 10.2% in the Republic of Korea, of which approximately 74.5% were diagnosed with AD [2]. Both reports showed that the incidence of AD increases with age and that the prevalence of AD is expected to increase until 2050. The cause of AD is not completely understood [3], and its pathophysiology is associated with amyloid-beta (Aβ) and tau protein accumulation, glial dysfunction, neurodegeneration (loss of neuronal connections), and altered oscillatory network activity [1,4-6].
- Approximately 70% of the risk of AD is believed to be inherited from, with many genes usually involved [7]. Glenner and Wong [8] first suggested a correlation between cerebrovascular Aβ protein and Down syndrome (trisomy 21), which is homologous to AD. In dominantly inherited AD, missense mutations in amyloid precursor protein (APP) or presenilin-1/-2 genes on chromosome 21 increase Aβ production. Nondominant AD increases Aβ levels in the brain via the failure of Aβ clearance. Both of these situations result in the accumulation of Aβ42 oligomers in limbic systems. Affluent diffuse Aβ plaques without neuritic dystrophy, microgliosis, astrocytosis, and tangle formation have been observed in people who died in their early to mid-teens because of familial AD [9]. Aβ42 oligomers, which have been isolated from late-onset AD brains, reduce synapse density, suppress prolonged potentiation, and reinforce prolonged synaptic depression in the rodent hippocampus [9], and intraventricular injection of Aβ42 oligomers damages memory in healthy mature rats [10].
- Thus, Aβ could directly or indirectly injure synapses and induce neuritis [9]. Aβ42 oligomers in patients with AD could also induce tau phosphorylation, which is associated with an increase in neurofibrillary tangles and neurotoxicity [9,11]. Since the discovery of Aβ protein, the Aβ hypothesis [12,13] has become the dominant model of AD pathogenesis and is guiding the development of potential therapeutic strategies. Although it is unclear how Aβ accumulates in the central nervous system and subsequently initiates AD, the generation of Aβ may occur in the neuronal axonal membranes after APP-mediated axonal transport of β-secretase, γ-secretase, and presenilin-1 [14,15], thus forming senile plaques outside neurons [16,17].
- According to the Aβ hypothesis, several strategies have been identified as possible interventions against Aβ [18], including inhibitors against β-secretase or γ-secretase, selective Aβ42-lowering agents, and immunotherapy against Aβ. The results of a few clinical trials with monoclonal antibodies to Aβ have suggested a significant cognitive decline in patients with mild, but not moderate AD [9], but most immunotherapies eventually failed in phase II (crenezumab and gantenerumab) or phase III (solanezumab, aducanumab, and bapineuzumab) clinical trials [19]. These failures of Aβ monoclonal antibodies imply the need for a new approach to treat patients with AD.
- The ion channel hypothesis postulates that oligomers of soluble, nonfibrillar Aβ form membrane ion channels, allowing the unregulated calcium influx into neurons [20,21] that underlies the disrupted calcium ion homeostasis and apoptosis seen in AD [22]. Optogenetics is a neuromodulation method that uses a combination of genetic methods and optical instruments to allow light to modulate the specific molecular and cellular activities of individual neurons in living tissue [23-26]. In this review, we will discuss the historical applications of optogenetics to investigate the mechanisms and possible therapeutic strategies involved in AD based on the Aβ hypothesis.
Introduction
- After Crick [27] speculated the concept of using light to control neuronal activity in 1979, Callaway and Katz [28] used light to uncage glutamine in living brain slices. Zemelman et al. [29] developed a targeting method using light to control rhodopsin-sensitized neurons. Nagel et al. [30] first applied the optogenetic manipulation of cation-selective ion movement by expressing channelrhodopsin-2 in Xenopus laevis and mammalian cells. Boyden et al. [23] used channelrhodopsin-2 to control neuronal spiking and synaptic transmission.
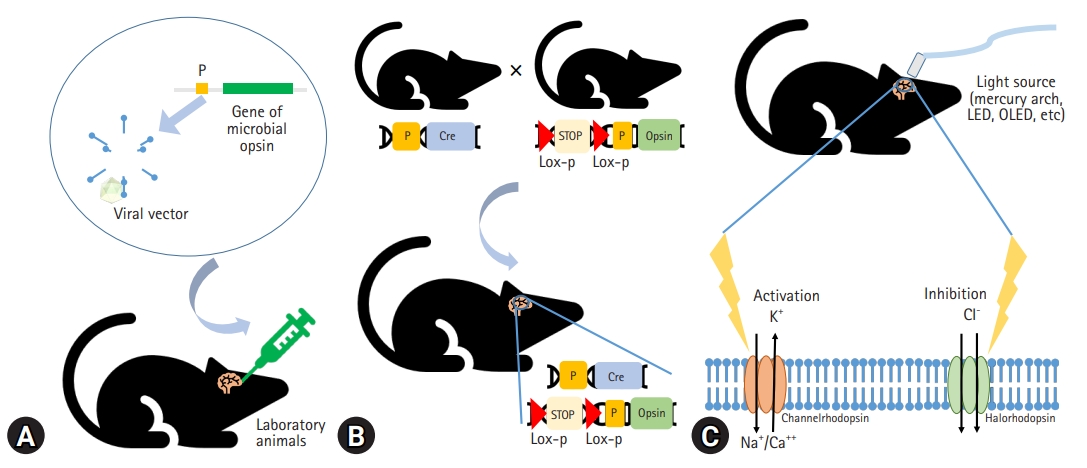
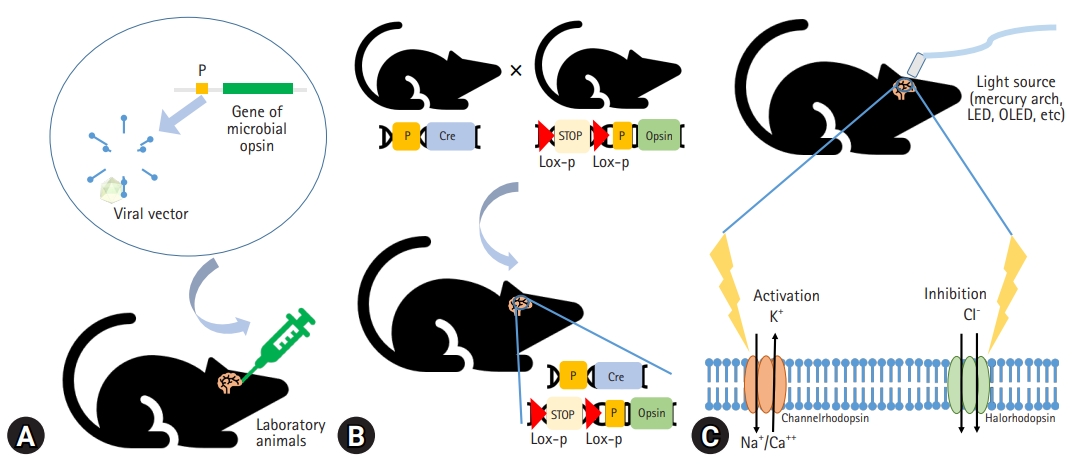
- Based on this historical background, the application of optogenetics is fundamentally composed of (1) light-sensitive microbial opsin engineering, (2) genetic methods to introduce the opsin into cells, and (3) optical instruments for guiding light to activate or inhibit specific neural circuits to manipulate their behavior with temporal precision [23,25,31] (Fig. 1).
- Light-activated proteins are required for the optical manipulation of molecular or cellular activity. Channelrhodopsin and anion-conducting channelrhodopsins are used to excite and inhibit neurons, respectively [32]. Halorhodopsin, bacteriorhodopsin, and archaerhodopsins are also used to inhibit neuronal activity [33,34].
- The expression of microbial opsins in mammalian cells has been challenging. The use of viral vectors such as adeno-associated virus (AAV) is a fundamental method to express high levels of opsins, and the transfected neurons become electrically active in response to light [35,36]. Transgenic mice, including those using the Thy1 promoter, express opsins in the affected region at higher specificity than viral vectors do [36,37]. Using the Cre/lox recombinase system to create transgenic mice is a novel approach to optogenetics [36]. Photo-activable Cre recombinase can stably modify gene expression in the mouse brain [38,39].
- Optogenetics principally depends on light stimulation. Although mercury arc lamps, light-emitting diodes (LEDs), and lasers have been used as in vitro light sources, organic LEDs are emerging technologies for optogenetics. Organic LEDs are suitable for implantation into the brain because they are softer, thinner, and more flexible than existing light sources and can supply adequate optical power over an acceptable temperature range [40]. Eventually, optogenetic techniques allow localized modulation of cell types of interest and simultaneous bidirectional control [41]. Moreover, the amplitude of stimulation and the time course are easily controlled by the light. This stimulation was shown to be relatively reproducible [42].
Optogenetic technique as a new neuromodulatory method
- The different cell types in the central nervous system interact with each other, resulting in specific rhythmic oscillations such as delta, theta, alpha, beta, gamma, and sharp-wave ripples [6]. Jasper and Andrews [43] first introduced the term “gamma wave” in their report on the “normal differentiation of occipital and precentral regions in man” to describe higher frequencies (35–48 Hz) beyond the beta range. The widely reported frequency of gamma oscillations is 25 to 140 Hz, with the 40-Hz frequency being of particular interest [44]. In addition to light, sound, or tactile stimuli [44], various methods to stimulate gamma waves, including temporal interference [45], ultrasound stimulations [46], and optogenetics [47] have been suggested. Gamma oscillations correlate with various functions of the brain, including sensory processing and cognitive functions such as learning and memory [48]. Inter-areal coherence and local regulation have generated interest in gamma oscillations [49,50]. Parvalbumin-positive inhibitory neurons are dominant in gamma oscillation generation [6,51], while the activation of pyramidal neurons increases lower frequency oscillations in vivo [49].
- Decreased synchronization of gamma oscillations [52-54] or enhanced gamma band power and lagged gamma responses [55,56] have been observed in patients with AD. Disturbances of slow gamma oscillations have also been reported in rodent AD models [57]. Interestingly, the transgenic APP-presenilin-1 mouse model of AD exhibits decreased gamma oscillation power in the lateral entorhinal cortex, which transmits various sensory inputs to the hippocampus and thus participates in memory processes analogous to those affected by human AD [58]. Decreased hippocampal slow gamma oscillation power has also been observed in a transgenic mouse model of AD [57].
- Stimulation of gamma oscillations may have therapeutic potential for AD. Stimulation with light and sound sources at 1 to 30 Hz increases physical and cortical performance in patients with AD [59]. Light and sound stimulation between 8 and 15 Hz in patients with AD who are elderly improves cognitive performance, memory function, and alpha waves [44,60]. Visual stimulation by light flashes increases gamma band activity, in which patients with AD demonstrate increased frontoparietal gamma coherence and reduced occipitoparietal coherence [44,56].
- Although the precise molecular and cellular mechanisms by which gamma oscillation stimulation ameliorates AD pathology are unknown, a correlation between Aβ and altered gamma oscillations has been reported. Decreased gamma oscillations could appear without Aβ plaques in TAS10 mice overexpressing human APP [61]. A close association between reduced gamma activity and functional behavioral deficits, as well as altered hippocampal gamma oscillations connected to Aβ, was found in the olfactory network of APPswe transgenic mice [62].
Gamma oscillation entrainment and Alzheimer disease
- As the control of neural activity and neural circuit interrogation was made possible using optogenetic techniques [35,63], optogenetic approaches to AD subsequently began.
- Since the loss of α4β2 nicotinic receptors is increased in AD [64-67], acetylcholine is released synaptically by optogenetic stimulation [68]. Bell et al. [68] suggested that activation of α4β2 receptors mediates nicotinic excitatory postsynaptic potential (EPSP) in CA1 interneurons by affecting the stratum lacunosum-moleculare using retroviral AAV expressing oChIEF in a Cre-dependent manner. Optogenetic activation of pyramidal neurons in the entorhinal cortex layer III improves synaptic defects between pyramidal neurons and CA1 parvalbumin-positive neurons in transgenic AD mice. It also halts the decrease in spatial learning and memory [69]. Although AAV has been generally used as a viral vector, the incidence of sharp wave ripples is reduced by optogenetic stimulation at the target location. The medial septum cholinergic stimulation of sleeping animals decreases sharp-wave ripples and advances theta-gamma oscillations. This research highlights the significance of the timing of cholinergic input. This could explain the limited success of cholinesterase inhibitor drugs in AD [70].
- Optogenetic inhibition of hilar GABAergic interneurons of the dentate gyrus (DG) through Cre-dependent gene expression of enhanced halorhodopsin disrupts spatial learning and memory retrieval without affecting short-term working memory, motor coordination, and memory retention. Using optogenetic stimulation, GABAergic interneurons can be activated without affecting pyramidal neurons in the CA3 and CA1 regions [71]. Optogenetic stimulation of hippocampal memory engram cells in transgenic AD mice overexpressing APP/presenilin-1 induces memory retrieval. Optogenetic stimulation of DG engram cells improved long-term memory and spine density [72]. Optogenetic stimulation of the DG in APP/presenilin-1 × ArcCreERT2 × channelrhodopsin-2-enhanced yellow fluorescent protein mice improved memory impairment. Stimulation of DG neural ensembles leads to enhancement of memory retrieval and reactivation of neural ensembles [73], which suggests that optogenetic DG manipulation could be a target for AD treatment.
- Optogenetic activation of glutamatergic neurons in Aβ-injected mice improves working memory and short-term memory without affecting long-term memory in the bilateral DG. This stimulation downregulates Aβ and upregulates neuronal nuclei, which are biomarkers of neuroprotection [10]. As antagonism of adenosine A2A receptor (A2AR) mimics memory impairment prevention in AD animal models [74-77], optogenetic activation of a chimeric rhodopsin-adenosine A2AR protein activates cyclic adenosine monophosphate (cAMP) signaling, which increases cAMP levels and mitogen-activated protein kinase phosphorylation. This activation induces memory dysfunction in the hippocampus through phospho-CREB signaling [77]. These reports suggest that multiple, targeted optogenetic approaches can be used to treat AD [10].
Optogenetic neuromodulation and Alzheimer disease
- Since the excitation of gamma oscillations reduces circuit noise and amplifies signals that result in an increase in the signal transmission of the neocortex [49], optogenetics-induced gamma oscillations may have therapeutic potential for AD. Studies on the applications of optogenetics to 40-Hz gamma oscillations have been ongoing since the optogenetic stimulation of fast-spiking parvalbumin-positive interneurons in gamma oscillations was first demonstrated in mice [78]. Entrainment or synchronization of hippocampal gamma oscillations and spiking to 40 Hz via noninvasive stimuli, such as flashing lights or pulses of sound [79], reduces the Aβ load and activates microglia in a well-established 5XFAD mouse model of AD [80].
- Decreased amyloidogenesis and increased amyloid endocytosis can be mediated by microglia [80]. Co-localization of microglia and Aβ was confirmed by histological analysis and induction of genes related to morphologic transformation of microglia was confirmed by gene expression profiling. That study suggested a neuroprotective role of gamma oscillations that affect neurons and microglia. Gamma oscillations also decrease phosphorylated tau protein levels [80].
- In the JA20 AD mouse model, optogenetic stimulation of parvalbumin-positive interneurons restores slow gamma oscillations and increases spatial memory [47]. Accumulation of Aβ1-42 oligomers disrupts long-term potential and theta-nested gamma oscillations in the hippocampus. Furthermore, stimulation of GABAergic interneurons reduces neuroinflammation and activates autophagy. Photostimulated APP/presenilin-1 mice showed a significant decrease in escape latency in the Morris water maze test, indicating that optogenetic stimulation ameliorates spatial learning [81]. Optogenetic modulation of channelrhodopsin-2-expressing parvalbumin-positive interneurons restores gamma oscillations and gamma oscillation-induced spike timing-dependent long-term potentiation [82]. This activation selectively increases spontaneous inhibitory postsynaptic currents at theta and gamma frequencies and restores Aβ-induced reductions [83].
- However, activation of parvalbumin-positive neurons by 40-Hz optical stimulation in the basal forebrain increased Aβ1-42 levels. Accumulation of amyloid plaques was increased in the medial prefrontal cortex and the septal nuclei. These results indicate that the method of activation of gamma oscillations changes the modulation of Aβ plaques [84]. Optogenetic stimulation of double-frequency slow waves increased the disruption of calcium homeostasis by Aβ and induced synaptic spine loss [85]. Subsequent human clinical trials of gamma oscillation band stimulation have shown mild cognitive improvements in patients with AD who have been exposed to light, sound, or tactile stimuli in the 40-Hz range [44]. However, the precise molecular and cellular mechanisms by which gamma oscillation band stimulation ameliorates AD pathology are unknown.
Optogenetics-induced gamma oscillations and Alzheimer disease
- Various anti-Aβ therapies are ongoing in clinical trials, but effective drugs are still lacking [86]. Although optogenetic technology for AD could be a new therapeutic approach, the major limitation of optogenetics is the use of viral vectors to express microbial opsins in human cells. Using viral vectors for gene therapy is considered a risky method that has not been fully tested to date, since AAV may cause activation of innate immunity and systemic inflammatory responses in humans [87,88]. Current optogenetics is mostly invasive because of the implantation of optic fibers, and overheating that induces tissue damage may be caused by the light [89]. Optogenetic stimulation also increases neuronal DNA double-strand breaks in mice [90]. The inappropriate use of optogenetics may paradoxically induce AD. Five months of chronic optogenetic stimulation could increase the formation of Aβ [91] and the release of tau protein [92]. Moreover, it remains a challenge to target opsins to defined organelles, including the plasma membrane or mitochondria [93,94] or to specific regions including dendrites or axon terminals [94].
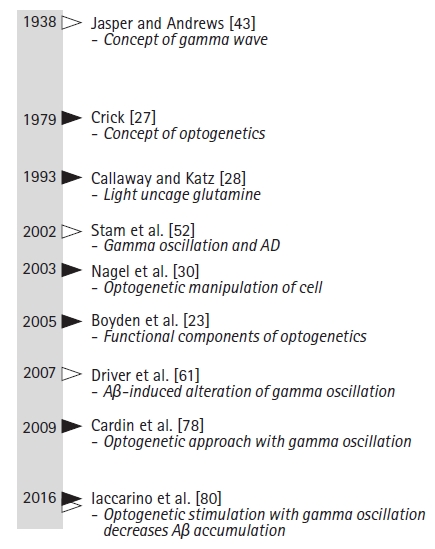
- Although optogenetics may have limitations, optogenetic neuromodulation allows for deep brain stimulation. In addition to AD, optogenetics-driven research has led to insights into Parkinson disease [93,95], autism, schizophrenia, drug abuse, anxiety, and depression [34,49,78,96]. As shown in the historical timeline (Fig. 2), this technology could modulate specific targets and neuronal activity [97]. The technical development of light delivery sources is also required. MicroLED arrays selectively stimulate opsins and act as biological amplifiers [98]. For in vivo modulation, the wireless form of a light source improves the application of optogenetics. Wireless control of light sources has been studied since 2011 [99]. In vivo injectable instruments require safe injectable battery technologies. The battery-free wireless system developed by Zhang et al. [100] could be another solution.
Limitations and prospects of optogenetics
- A new clinical approach for AD is needed because of the failure of Aβ monoclonal antibodies. Optogenetics could play key roles in learning the mechanisms of cellular responses and thus has the potential to treat neuronal diseases. In addition, optogenetics-induced gamma oscillations might provide a new method to modulate local neuronal signals in AD. Further research is needed to determine how optogenetics might be associated with gamma oscillations, and we suggest that, based on studies to date, it is highly related to the continuity of excitation-inhibition signals, frequency of gamma oscillations, and cytokine production-related cell signaling. Although optogenetics and gamma oscillations are currently not fundamental therapeutic approaches for AD, their combination could be a new way to manage AD. The development of actuators and sensors must precede the clinical use of optogenetics, since the viral vectors and opsins that have been used in optogenetic research are currently limited. As deep learning technology advances, the artificial manufacturing of opsins or modulation of viral vectors could be a breakthrough in optogenetic technology.
Conclusion
-
Conflicts of interest
No potential conflicts of interest relevant to this article was reported.
-
Funding
This work was supported by the 2022 education, research and student guidance grant funded by Jeju National University.
-
Author contributions
Conceptualization: HK, SPY; Data Curation: HK; Funding acquisition: SPY; Supervision: SPY; Writing-original draft: HK; Writing-review & editing: SPY.
Notes

- 1. 2021 Alzheimer’s disease facts and figures. Alzheimers Dement 2021;17:327–406.PubMed
- 2. Lee JS, Kang MJ, Nam HJ, Kim YJ, Lee OJ, Kim KO. Korean dementia observatory 2019 (Report No. NIDR-1902-0028) [Internet]. Seoul: Central Dementia Center Service; 2020 [cited 2021 Dec 09]. https://www.nid.or.kr/info/dataroom_view.aspx?bid=209.
- 3. Burns A, Iliffe S. Alzheimer’s disease. BMJ 2009;338:b158.ArticlePubMed
- 4. Nimmrich V, Draguhn A, Axmacher N. Neuronal network oscillations in neurodegenerative diseases. Neuromolecular Med 2015;17:270–84.ArticlePubMed
- 5. Canter RG, Penney J, Tsai LH. The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature 2016;539:187–96.ArticlePubMed
- 6. Strüber D, Herrmann CS. Modulation of gamma oscillations as a possible therapeutic tool for neuropsychiatric diseases: a review and perspective. Int J Psychophysiol 2020;152:15–25.ArticlePubMed
- 7. Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer’s disease. Lancet 2011;377:1019–31.ArticlePubMed
- 8. Glenner GG, Wong CW. Alzheimer’s disease and Down’s syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun 1984;122:1131–5.ArticlePubMed
- 9. Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 2016;8:595–608.ArticlePubMedPMC
- 10. Cui X, Zhang F, Zhang H, Huang X, Wang K, Huang T, et al. Neuroprotective effect of optogenetics varies with distance from channelrhodopsin-2 expression in an amyloid-β-injected mouse model of Alzheimer’s disease. Front Neurosci 2020;14:583628.ArticlePubMedPMC
- 11. Gao Y, Tan L, Yu JT, Tan L. Tau in Alzheimer’s disease: mechanisms and therapeutic strategies. Curr Alzheimer Res 2018;15:283–300.ArticlePubMed
- 12. Selkoe DJ. The molecular pathology of Alzheimer’s disease. Neuron 1991;6:487–98.ArticlePubMed
- 13. Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science 1992;256:184–5.ArticlePubMed
- 14. Kamal A, Almenar-Queralt A, LeBlanc JF, Roberts EA, Goldstein LS. Kinesin-mediated axonal transport of a membrane compartment containing beta-secretase and presenilin-1 requires APP. Nature 2001;414:643–8.ArticlePubMed
- 15. Hooper NM. Roles of proteolysis and lipid rafts in the processing of the amyloid precursor protein and prion protein. Biochem Soc Trans 2005;33(Pt 2):335–8.ArticlePubMed
- 16. Ohnishi S, Takano K. Amyloid fibrils from the viewpoint of protein folding. Cell Mol Life Sci 2004;61:511–24.ArticlePubMed
- 17. Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J. The importance of neuritic plaques and tangles to the development and evolution of AD. Neurology 2004;62:1984–9.ArticlePubMed
- 18. Citron M. Strategies for disease modification in Alzheimer’s disease. Nat Rev Neurosci 2004;5:677–85.ArticlePubMed
- 19. Oxford AE, Stewart ES, Rohn TT. Clinical trials in Alzheimer’s disease: a hurdle in the path of remedy. Int J Alzheimers Dis 2020;2020:5380346.ArticlePubMedPMC
- 20. Arispe N, Rojas E, Pollard HB. Alzheimer disease amyloid beta protein forms calcium channels in bilayer membranes: blockade by tromethamine and aluminum. Proc Natl Acad Sci U S A 1993;90:567–71.ArticlePubMedPMC
- 21. Ekinci FJ, Linsley MD, Shea TB. Beta-amyloid-induced calcium influx induces apoptosis in culture by oxidative stress rather than tau phosphorylation. Brain Res Mol Brain Res 2000;76:389–95.ArticlePubMed
- 22. Abramov AY, Canevari L, Duchen MR. Calcium signals induced by amyloid beta peptide and their consequences in neurons and astrocytes in culture. Biochim Biophys Acta 2004;1742:81–7.ArticlePubMed
- 23. Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 2005;8:1263–8.ArticlePubMed
- 24. Deisseroth K, Feng G, Majewska AK, Miesenböck G, Ting A, Schnitzer MJ. Next-generation optical technologies for illuminating genetically targeted brain circuits. J Neurosci 2006;26:10380–6.ArticlePubMedPMC
- 25. Tye KM, Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci 2012;13:251–66.ArticlePubMedPMC
- 26. Byun J. Optogenetics: a new frontier for cell physiology study. J Life Sci 2015;25:953–9.Article
- 27. Crick F. The impact of molecular biology on neuroscience. Philos Trans R Soc Lond B Biol Sci 1999;354:2021–5.ArticlePubMedPMC
- 28. Callaway EM, Katz LC. Photostimulation using caged glutamate reveals functional circuitry in living brain slices. Proc Natl Acad Sci U S A 1993;90:7661–5.ArticlePubMedPMC
- 29. Zemelman BV, Lee GA, Ng M, Miesenböck G. Selective photostimulation of genetically chARGed neurons. Neuron 2002;33:15–22.ArticlePubMed
- 30. Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A 2003;100:13940–5.ArticlePubMedPMC
- 31. Pama EA, Colzato LS, Hommel B. Optogenetics as a neuromodulation tool in cognitive neuroscience. Front Psychol 2013;4:610.ArticlePubMedPMC
- 32. Beck S, Yu-Strzelczyk J, Pauls D, Constantin OM, Gee CE, Ehmann N, et al. Synthetic light-activated ion channels for optogenetic activation and inhibition. Front Neurosci 2018;12:643.ArticlePubMedPMC
- 33. Gradinaru V, Thompson KR, Deisseroth K. eNpHR: a Natronomonas halorhodopsin enhanced for optogenetic applications. Brain Cell Biol 2008;36:129–39.ArticlePubMedPMC
- 34. Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, et al. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science 2010;330:1677–81.ArticlePubMedPMC
- 35. Zhang F, Gradinaru V, Adamantidis AR, Durand R, Airan RD, de Lecea L, et al. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat Protoc 2010;5:439–56.ArticlePubMedPMC
- 36. Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci 2011;34:389–412.ArticlePubMedPMC
- 37. Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, Augustine GJ, et al. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 2007;54:205–18.ArticlePubMedPMC
- 38. Schindler SE, McCall JG, Yan P, Hyrc KL, Li M, Tucker CL, et al. Photo-activatable Cre recombinase regulates gene expression in vivo. Sci Rep 2015;5:13627.ArticlePubMedPMC
- 39. Kawano F, Okazaki R, Yazawa M, Sato M. A photoactivatable Cre-loxP recombination system for optogenetic genome engineering. Nat Chem Biol 2016;12:1059–64.ArticlePubMed
- 40. Matarèse BF, Feyen PL, de Mello JC, Benfenati F. Sub-millisecond control of neuronal firing by organic light-emitting diodes. Front Bioeng Biotechnol 2019;7:278.ArticlePubMedPMC
- 41. Mahmoudi P, Veladi H, Pakdel FG. Optogenetics, tools and applications in neurobiology. J Med Signals Sens 2017;7:71–9.ArticlePubMedPMC
- 42. Malyshev A, Goz R, LoTurco JJ, Volgushev M. Advantages and limitations of the use of optogenetic approach in studying fast-scale spike encoding. PLoS One 2015;10:e0122286.ArticlePubMedPMC
- 43. Jasper HH, Andrews HL. Electro-encephalography: III. Normal differentiation of occipital and precentral regions in man. Arch Neurol Psychiatr 1938;39:96–115.Article
- 44. McDermott B, Porter E, Hughes D, McGinley B, Lang M, O’Halloran M, et al. Gamma band neural stimulation in humans and the promise of a new modality to prevent and treat Alzheimer’s disease. J Alzheimers Dis 2018;65:363–92.ArticlePubMedPMC
- 45. Esmaeilpour Z, Kronberg G, Reato D, Parra LC, Bikson M. Temporal interference stimulation targets deep brain regions by modulating neural oscillations. Brain Stimul 2021;14:55–65.ArticlePubMed
- 46. Yuan Y, Yan J, Ma Z, Li X. Effect of noninvasive focused ultrasound stimulation on gamma oscillations in rat hippocampus. Neuroreport 2016;27:508–15.ArticlePubMed
- 47. Etter G, van der Veldt S, Manseau F, Zarrinkoub I, Trillaud-Doppia E, Williams S. Optogenetic gamma stimulation rescues memory impairments in an Alzheimer’s disease mouse model. Nat Commun 2019;10:5322.ArticlePubMedPMC
- 48. Herrmann CS, Fründ I, Lenz D. Human gamma-band activity: a review on cognitive and behavioral correlates and network models. Neurosci Biobehav Rev 2010;34:981–92.ArticlePubMed
- 49. Sohal VS. How close are we to understanding what (if anything) γ oscillations do in cortical circuits? J Neurosci 2016;36:10489–95.ArticlePubMedPMC
- 50. Adaikkan C, Tsai LH. Gamma entrainment: impact on neurocircuits, glia, and therapeutic opportunities. Trends Neurosci 2020;43:24–41.ArticlePubMed
- 51. Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 2007;8:45–56.ArticlePubMed
- 52. Stam CJ, van Cappellen van Walsum AM, Pijnenburg YA, Berendse HW, de Munck JC, Scheltens P, et al. Generalized synchronization of MEG recordings in Alzheimer’s disease: evidence for involvement of the gamma band. J Clin Neurophysiol 2002;19:562–74.ArticlePubMed
- 53. Stam CJ, van der Made Y, Pijnenburg YA, Scheltens P. EEG synchronization in mild cognitive impairment and Alzheimer’s disease. Acta Neurol Scand 2003;108:90–6.ArticlePubMed
- 54. Koenig T, Prichep L, Dierks T, Hubl D, Wahlund LO, John ER, et al. Decreased EEG synchronization in Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging 2005;26:165–71.ArticlePubMed
- 55. van Deursen JA, Vuurman EF, Verhey FR, van Kranen-Mastenbroek VH, Riedel WJ. Increased EEG gamma band activity in Alzheimer’s disease and mild cognitive impairment. J Neural Transm (Vienna) 2008;115:1301–11.ArticlePubMedPMC
- 56. Başar E, Emek-Savaş DD, Güntekin B, Yener GG. Delay of cognitive gamma responses in Alzheimer’s disease. Neuroimage Clin 2016;11:106–15.ArticlePubMedPMC
- 57. Mably AJ, Colgin LL. Gamma oscillations in cognitive disorders. Curr Opin Neurobiol 2018;52:182–7.ArticlePubMedPMC
- 58. Klein AS, Donoso JR, Kempter R, Schmitz D, Beed P. Early cortical changes in Gamma oscillations in Alzheimer’s disease. Front Syst Neurosci 2016;10:83.ArticlePubMedPMC
- 59. da Silva VF, Ribeiro AP, Dos Santos VA, Nardi AE, King AL, Calomeni MR. Stimulation by light and sound: therapeutics effects in humans: systematic review. Clin Pract Epidemiol Ment Health 2015;11:150–4.ArticlePubMedPMC
- 60. Calomeni MR, Furtado da Silva V, Velasques BB, Feijó OG, Bittencourt JM, Ribeiro de Souza E Silva AP. Modulatory effect of association of brain stimulation by light and binaural beats in specific brain waves. Clin Pract Epidemiol Ment Health 2017;13:134–44.ArticlePubMedPMC
- 61. Driver JE, Racca C, Cunningham MO, Towers SK, Davies CH, Whittington MA, et al. Impairment of hippocampal gamma-frequency oscillations in vitro in mice overexpressing human amyloid precursor protein (APP). Eur J Neurosci 2007;26:1280–8.ArticlePubMed
- 62. Peña-Ortega F. Amyloid beta-protein and neural network dysfunction. J Neurodegener Dis 2013;2013:657470.ArticlePubMedPMC
- 63. Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron 2011;71:9–34.ArticlePubMed
- 64. Kellar KJ, Whitehouse PJ, Martino-Barrows AM, Marcus K, Price DL. Muscarinic and nicotinic cholinergic binding sites in Alzheimer’s disease cerebral cortex. Brain Res 1987;436:62–8.ArticlePubMed
- 65. Marutle A, Warpman U, Bogdanovic N, Nordberg A. Regional distribution of subtypes of nicotinic receptors in human brain and effect of aging studied by (+/-)-[3H]epibatidine. Brain Res 1998;801:143–9.ArticlePubMed
- 66. Perry DC, Dávila-García MI, Stockmeier CA, Kellar KJ. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J Pharmacol Exp Ther 1999;289:1545–52.PubMed
- 67. Perry E, Martin-Ruiz C, Lee M, Griffiths M, Johnson M, Piggott M, et al. Nicotinic receptor subtypes in human brain ageing, Alzheimer and Lewy body diseases. Eur J Pharmacol 2000;393:215–22.ArticlePubMed
- 68. Bell KA, Shim H, Chen CK, McQuiston AR. Nicotinic excitatory postsynaptic potentials in hippocampal CA1 interneurons are predominantly mediated by nicotinic receptors that contain α4 and β2 subunits. Neuropharmacology 2011;61:1379–88.ArticlePubMedPMC
- 69. Yang X, Yao C, Tian T, Li X, Yan H, Wu J, et al. A novel mechanism of memory loss in Alzheimer’s disease mice via the degeneration of entorhinal-CA1 synapses. Mol Psychiatry 2018;23:199–210.ArticlePubMed
- 70. Jarzebowski P, Tang CS, Paulsen O, Hay YA. Impaired spatial learning and suppression of sharp wave ripples by cholinergic activation at the goal location. Elife 2021;10:e65998.ArticlePubMedPMC
- 71. Andrews-Zwilling Y, Gillespie AK, Kravitz AV, Nelson AB, Devidze N, Lo I, et al. Hilar GABAergic interneuron activity controls spatial learning and memory retrieval. PLoS One 2012;7:e40555.ArticlePubMedPMC
- 72. Roy DS, Arons A, Mitchell TI, Pignatelli M, Ryan TJ, Tonegawa S. Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature 2016;531:508–12.ArticlePubMedPMC
- 73. Perusini JN, Cajigas SA, Cohensedgh O, Lim SC, Pavlova IP, Donaldson ZR, et al. Optogenetic stimulation of dentate gyrus engrams restores memory in Alzheimer’s disease mice. Hippocampus 2017;27:1110–22.ArticlePubMedPMC
- 74. Arendash GW, Schleif W, Rezai-Zadeh K, Jackson EK, Zacharia LC, Cracchiolo JR, et al. Caffeine protects Alzheimer’s mice against cognitive impairment and reduces brain beta-amyloid production. Neuroscience 2006;142:941–52.ArticlePubMed
- 75. Dall’Igna OP, Fett P, Gomes MW, Souza DO, Cunha RA, Lara DR. Caffeine and adenosine A(2a) receptor antagonists prevent beta-amyloid (25-35)-induced cognitive deficits in mice. Exp Neurol 2007;203:241–5.ArticlePubMed
- 76. Cunha GM, Canas PM, Melo CS, Hockemeyer J, Müller CE, Oliveira CR, et al. Adenosine A2A receptor blockade prevents memory dysfunction caused by beta-amyloid peptides but not by scopolamine or MK-801. Exp Neurol 2008;210:776–81.ArticlePubMed
- 77. Li P, Rial D, Canas PM, Yoo JH, Li W, Zhou X, et al. Optogenetic activation of intracellular adenosine A2A receptor signaling in the hippocampus is sufficient to trigger CREB phosphorylation and impair memory. Mol Psychiatry 2015;20:1339–49.ArticlePubMedPMC
- 78. Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 2009;459:663–7.ArticlePubMedPMC
- 79. Thomson H. How flashing lights and pink noise might banish Alzheimer’s, improve memory and more. Nature 2018;555:20–2.Article
- 80. Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 2016;540:230–5.ArticlePubMedPMC
- 81. Zhang Z, Jing Y, Ma Y, Duan D, Li B, Hölscher C, et al. Driving GABAergic neurons optogenetically improves learning, reduces amyloid load and enhances autophagy in a mouse model of Alzheimer’s disease. Biochem Biophys Res Commun 2020;525:928–35.ArticlePubMed
- 82. Park K, Lee J, Jang HJ, Richards BA, Kohl MM, Kwag J. Optogenetic activation of parvalbumin and somatostatin interneurons selectively restores theta-nested gamma oscillations and oscillation-induced spike timing-dependent long-term potentiation impaired by amyloid β oligomers. BMC Biol 2020;18:7.ArticlePubMedPMC
- 83. Chung H, Park K, Jang HJ, Kohl MM, Kwag J. Dissociation of somatostatin and parvalbumin interneurons circuit dysfunctions underlying hippocampal theta and gamma oscillations impaired by amyloid β oligomers in vivo. Brain Struct Funct 2020;225:935–54.ArticlePubMedPMC
- 84. Wilson CA, Fouda S, Sakata S. Effects of optogenetic stimulation of basal forebrain parvalbumin neurons on Alzheimer’s disease pathology. Sci Rep 2020;10:15456.ArticlePubMedPMC
- 85. Kastanenka KV, Calvo-Rodriguez M, Hou SS, Zhou H, Takeda S, Arbel-Ornath M, et al. Frequency-dependent exacerbation of Alzheimer’s disease neuropathophysiology. Sci Rep 2019;9:8964.ArticlePubMedPMC
- 86. Huang LK, Chao SP, Hu CJ. Clinical trials of new drugs for Alzheimer disease. J Biomed Sci 2020;27:18.ArticlePubMedPMC
- 87. Marshall E. Gene therapy death prompts review of adenovirus vector. Science 1999;286:2244–5.ArticlePubMed
- 88. Kim GU, Kim HI, Chung E. Towards human clinical application of emerging optogenetics technology. Biomed Eng Lett 2011;1:207–12.Article
- 89. Bostancıklıoğlu M. An update on memory formation and retrieval: an engram-centric approach. Alzheimers Dement 2020;16:926–37.ArticlePubMed
- 90. Suberbielle E, Sanchez PE, Kravitz AV, Wang X, Ho K, Eilertson K, et al. Physiologic brain activity causes DNA double-strand breaks in neurons, with exacerbation by amyloid-β. Nat Neurosci 2013;16:613–21.ArticlePubMedPMC
- 91. Yamamoto K, Tanei ZI, Hashimoto T, Wakabayashi T, Okuno H, Naka Y, et al. Chronic optogenetic activation augments aβ pathology in a mouse model of Alzheimer disease. Cell Rep 2015;11:859–65.ArticlePubMed
- 92. Wu JW, Hussaini SA, Bastille IM, Rodriguez GA, Mrejeru A, Rilett K, et al. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat Neurosci 2016;19:1085–92.ArticlePubMedPMC
- 93. Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science 2009;324:354–9.ArticlePubMedPMC
- 94. Zalocusky KA, Fenno LE, Deisseroth K. Current challenges in optogenetics. In: Hegemann P, Sigrist S, editors. Optogenetics. Berlin: De Gruyter; 2013. p. 23–34.
- 95. Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 2010;466:622–6.ArticlePubMedPMC
- 96. Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 2009;324:1080–4.ArticlePubMedPMC
- 97. Elkouzi A, Vedam-Mai V, Eisinger RS, Okun MS. Emerging therapies in Parkinson disease: repurposed drugs and new approaches. Nat Rev Neurol 2019;15:204–23.ArticlePubMedPMC
- 98. Berlinguer-Palmini R, Narducci R, Merhan K, Dilaghi A, Moroni F, Masi A, et al. Arrays of microLEDs and astrocytes: biological amplifiers to optogenetically modulate neuronal networks reducing light requirement. PLoS One 2014;9:e108689.ArticlePubMedPMC
- 99. Iwai Y, Honda S, Ozeki H, Hashimoto M, Hirase H. A simple head-mountable LED device for chronic stimulation of optogenetic molecules in freely moving mice. Neurosci Res 2011;70:124–7.ArticlePubMed
- 100. Zhang Y, Castro DC, Han Y, Wu Y, Guo H, Weng Z, et al. Battery-free, lightweight, injectable microsystem for in vivo wireless pharmacology and optogenetics. Proc Natl Acad Sci U S A 2019;116:21427–37.ArticlePubMedPMC
References
Figure & Data
References
Citations

- Modulating Proteasome Function with Polyphenol Metabolites: A Promising Therapeutic Avenue for Alzheimer's Disease
Nyerovwo Charity Okei
European Journal of Medical and Health Research.2024; 2(2): 16. CrossRef - A comprehensive review of optical fiber technologies in optogenetics and their prospective developments in future clinical therapies
Siyu Chen, Zhuo Wang, Kun Xiao, Benzhao He, Jing Zhao, Xin Yang, Qingqing Liu, Anuj K. Sharma, Arnaldo Leal Junior, Rui Min
Optics & Laser Technology.2024; 179: 111332. CrossRef - Exogenous AMPA downregulates gamma-frequency network oscillation in CA3 of rat hippocampal slices
Chengzhang Li, Zhenrong Li, Sihan Xu, Sanwei Jiang, Zhenli Ye, Bin Yu, Shixiang Gong, Junmei Li, Qilin Hu, Bingyan Feng, Mengmeng Wang, Chengbiao Lu
Scientific Reports.2023;[Epub] CrossRef - Light-Controlled Modulation and Analysis of Neuronal Functions
Carlo Matera, Piotr Bregestovski
International Journal of Molecular Sciences.2022; 23(21): 12921. CrossRef

 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine PubReader
PubReader ePub Link
ePub Link Cite
Cite