Indexed in: ESCI, Scopus, PubMed,
PubMed Central, CAS, DOAJ, KCI
PubMed Central, CAS, DOAJ, KCI
FREE article processing charge

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Volume 36(3); 2019 > Article
-
Original article
Feasibility and efficacy of coil embolization for middle cerebral artery aneurysms -
Jae Young Choi1
 , Chang Hwa Choi2
, Chang Hwa Choi2 , Jun Kyeung Ko2
, Jun Kyeung Ko2 , Jae Il Lee2
, Jae Il Lee2 , Chae Wook Huh2
, Chae Wook Huh2 , Tae Hong Lee3
, Tae Hong Lee3
-
Yeungnam University Journal of Medicine 2019;36(3):208-218.
DOI: https://doi.org/10.12701/yujm.2019.00192
Published online: April 25, 2019
1Department of Neurosurgery, Kosin University Gospel Hospital, Busan, Korea
2Department of Neurosurgery, Pusan National University Hospital, Busan, Korea
3Department of Diagnostic Radiology, Pusan National University Hospital, Busan, Korea
- Corresponding author: Chang Hwa Choi, Department of Neurosurgery, Pusan National University Hospital, 179, Gudeok-ro, Seo-gu, Busan 49241, Korea Tel: +82-51-240-7547, Fax: +82-51-244-0282, E-mail: chwachoi@pusan.ac.kr
• Received: January 9, 2019 • Revised: February 19, 2019 • Accepted: April 10, 2019
Copyright © 2019 Yeungnam University College of Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 7,485 Views
- 92 Download
- 2 Crossref
Abstract
-
Background
- The anatomy of middle cerebral artery (MCA) aneurysms has been noted to be unfavorable for endovascular treatment. The purpose of this study was to assess the feasibility and efficacy of coiling for MCA aneurysms.
-
Methods
- From January 2004 to December 2015, 72 MCA aneurysms (38 unruptured and 34 ruptured) in 67 patients were treated with coils. Treatment-related complications, clinical outcomes, and immediate and follow-up angiographic outcomes were retrospectively analyzed.
-
Results
- Aneurysms were located at the MCA bifurcation (n=60), 1st segment (M1, n=8), and 2nd segment (M2, n=4). Sixty-nine aneurysms (95.8%) were treated by neck remodeling techniques using multi-catheter (n=44), balloon (n=14), stent (n=8), or combination of these (n=3). Only 3 aneurysms were treated by single-catheter technique. Angiographic results were 66 (91.7%) complete, 5 (6.9%) remnant neck, and 1 (1.4%) incomplete occlusion. Procedural complications included aneurysm rupture (n=1), asymptomatic coil migration to the distal vessel (n=1), and acute thromboembolism (n=10) consisting of 8 asymptomatic and 2 symptomatic events. Treatment-related permanent morbidity and mortality rates were 4.5% and 3.0%, respectively. There was no bleeding on clinical follow-up (mean, 29 months; range, 6-108 months). Follow-up angiographic results (mean, 26 months; range, 6-96 months) in patients included 1 major and 3 minor recanalizations.
-
Conclusion
- Coiling of MCA aneurysms could be a technically feasible and clinically effective treatment strategy with acceptable angiographic and clinical outcomes. However, the safety and efficacy of this technique as compared to surgical clipping remains to be ascertained.
- The International Subarachnoid Aneurysm Trial (ISAT) data in 2002 led to a significant change in the treatment of intracranial aneurysms. Although the ISAT study had exclusively addressed ruptured intracranial aneurysms, their results were rapidly assimilated into the treatment of unruptured intracranial aneurysms [1].
- However, there is still controversy about the treatment strategy for middle cerebral artery (MCA) aneurysms and surgical clipping is the preferred strategy, because MCA aneurysms are proximal to the cerebral surface and require less brain retraction to access and expose [2-4]. Moreover, endovascular coil embolization was likely to be unfavorable because MCA aneurysms often have wide necks and incorporate branches. Although some recent studies have demonstrated that endovascular embolization is equivalent to surgical clipping for the treatment of selected MCA aneurysms, coiling of wide-necked MCA aneurysm still remains technically challenging [5-9].
- However, newly developed devices and advanced neck-remodeling techniques using multi-catheter, balloon, stent, or a combination of these permit endovascular treatment (EVT) of complex aneurysms [9-12].
- The aim of this study was to retrospectively analyze the feasibility and efficacy of EVT for ruptured and unruptured MCA aneurysms at a center where coiling is the first option considered.
Introduction
- From January 2004 to December 2015, EVT was performed on 72 MCA aneurysms in 67 patients. Informed written consent was obtained from all patients in this retrospective study and approved by the Institutional Review Board of Kosin University Gospel Hospital (KUGH 2019-03-009).
- Patient and aneurysm characteristics, angiographic and clinical outcomes, and follow-up were evaluated by the referring neurosurgeons and an interventional neuroradiologist. Decision regarding treatment type (clipping versus coiling) was made by a neurovascular team involving neurosurgeons and an interventional neuroradiologist after completion of initial digital subtraction angiography (DSA). In agreement with our neurovascular team, coiling was the first-line treatment for MCA aneurysms, unless associated with compressive hematoma requiring immediate surgical evacuation. An experienced interventional neuroradiologist determined the possibility of coil embolization for MCA aneurysms, taking into consideration their shape, size, length of the neck, and complexity. Wide-neck aneurysms were defined as having a large neck (more than 4 mm) or a dome-to-neck ratio less than 1.5. Complex MCA aneurysms were defined by using particular anatomic features, including a branch vessel arising from the aneurysm sac and wide neck aneurysm with parent vessel incorporation.
- Inclusion criteria for this study were (1) ruptured MCA aneurysm, (2) unruptured MCA aneurysm ≥7 mm in size, (3) unruptured MCA aneurysm <7 mm in size with risk factors for aneurysm rupture such as previous or family history of subarachnoid hemorrhage (SAH), presence of lobulation or daughter sac, increased size on follow-up study, or multiple intracranial aneurysms, (4) patient criteria including young age, long life expectancy, and patients’ preferred treatment modality, or (5) recurred MCA aneurysm after coiling or clipping. The ruptured patients group was classified according to the Hunt and Hess grading scale (HHGS) to determine the clinical severity of the SAH. The modified Rankin scale (mRS) score was used to assess the clinical results recorded at each patient’s last follow-up consultation.
- 1. Endovascular treatment
- Antiplatelet premedication was not routinely prescribed in the early study period. Most patients with unruptured aneurysms were pre-medicated with dual antiplatelet therapy (aspirin 100 mg, clopidogrel 75 mg once a day) for at least 5 days prior to the procedure according to the patients’ medical condition. But, we did not use prophylactic antiplatelet pre-medication for patients with acutely ruptured aneurysms.
- SAH patients were treated within 24 hours after aneurysm rupture. In all patients with unruptured aneurysm, the therapeutic procedures were performed during a second angiography session. Local anesthesia was administered to all of the patients and electrocardiogram, arterial oxygen saturation, and blood pressure were appropriately monitored. A percutaneous intra-arterial approach was used after a standard Seldinger method and a 6 F introducer sheath was placed in the femoral artery. The baseline activated clotting time (ACT) was obtained before the procedure. Then patients received systemic heparinization and a bolus injection of 3,000 to 5,000 IU heparin just before starting the therapeutic procedure. A booster of 1,000 IU heparin was administered every hour to provide an ACT of longer than 250 seconds or twice the baseline ACT during the procedure.
- A 6 F guiding catheter (Envoy; Cordis Endovascular, Miami Lakes, FL, USA) was positioned in the internal carotid artery (ICA). A 6 F Shuttle sheath was used in patients with tortuous aortic arch and carotid artery anatomy or in cases in which complex endovascular techniques were anticipated.
- In most cases, coiling was tried first with the conventional single-catheter technique. When a single-catheter technique failed to make a stable coil mesh or was not suitable due to aneurysm geometry, aneurysm neck remodeling techniques using a multi-catheter, balloon, stent, or a combination of these were used.
- Immediate angiographic results were classified according to the Raymond classification [13]. Complete occlusion was defined as occlusion of the entire aneurysm sac; neck remnant occlusion, as the minimal portion of the aneurysm neck region filled with contrast media; and incomplete occlusion, as the aneurysm dome filled with contrast media.
- Immediate clinical outcome was evaluated according to the mRS by the neurosurgeons and the interventional neuroradiologist. All patients underwent non-enhanced brain computed tomography (CT) for evaluation of possible hemorrhagic complications and were monitored postoperatively at the intensive care unit.
- After the procedure, 2,850 IU of low molecular weight heparin (nadroparin) were also administered subcutaneously twice or three times a day for at least 2 days. The patients with stents were medicated dual antiplatelet therapy (aspirin 100 mg, clopidogrel 75 mg once a day) for at least 6 months.
- 2. Follow-up angiographic and clinical outcomes
- Follow-up magnetic resonance angiography or DSA was performed 6 months after the procedure. To compare immediate and last follow-up angiographic results, we defined a three-grade scale using the Raymond classification scale [13] as follows: (1) stable or improved occlusion, (2) minor recanalization demonstrating a change from class 1 to class 2 at follow-up, requiring only additional follow-up imaging, and (3) major recanalization demonstrating a change from class 1 to class 3 or from class 2 to class 3 at follow-up, which required retreatment.
- Follow-up clinical outcome was assessed by the neurosurgeons according to the mRS at last follow-up. Patients were classified as having favorable (mRS, 0–2) versus unfavorable outcomes (mRs, 3–6).
Materials and methods
- Patient and aneurysm characteristics were summarized in Table 1. The 67 patients (40 women and 27 men) ranged in age from 23 to 82 years (mean, 58.8 years). Of the 72 MCA aneurysms, 38 were unruptured in 33 patients and 34 were ruptured in 34 patients. Five patients each had 2 MCA aneurysms at the opposite side. Of these, 1 patient presented with SAH. One unruptured aneurysm was a recurred aneurysm after coil embolization. Eight (11.1%) were located in the main trunk of the artery (M1 segment), 60 (83.3%) at the first major bifurcation, and only 4 at the distal of M2 (5.6%). Aneurysm diameters ranged 2–38.9 mm (mean, 6.8 mm) and aneurysm neck widths 1–11.9 mm (mean, 3.58 mm). HHGS in the ruptured group were grade I in 1, grade II in 7, grade III in 14, grade IV in 8, and grade V in 4 patients.
- Three aneurysms were treated by the single-catheter technique. Sixty-nine aneurysms (95.8%) were treated with neck remodeling techniques using multi-catheter (n=44, Fig. 1), balloon (n=14, Fig. 2), stent (n=8, Fig. 3), or combination of these (n=3).
- Immediate post-embolization control angiograms revealed complete occlusion in 66, neck remnant occlusion in 5, and incomplete occlusion in 1 aneurysm by the Raymond classification. In the 38 unruptured aneurysm patients, 37 aneurysms (97.4%) demonstrated complete occlusion and only 1 patient (2.6%) revealed residual neck. In contrast, the group of ruptured patients showed complete occlusion in 29 (85.3%), neck remnant in 4 (11.8%), and incomplete occlusion in 1 patient (2.9%).
- Procedural complications occurred in 12 (16.7%) of 72 aneurysms. Six of 38 unruptured aneurysms experienced complications, including procedural aneurysm rupture (n=1) and acute thromboembolism without neurological deterioration (n=5). Two of 5 acute thromboembolic patients experienced symptomatic acute stroke at 6 and 10 days after treatment. Six of 34 ruptured aneurysm patients experienced complications, including asymptomatic coil migration to the distal vessel (n=1) and acute thromboembolism (n=5) which consisted of 3 asymptomatic embolic infarctions and 2 symptomatic acute strokes.
- One unruptured wide-necked aneurysm (5 mm in diameter) was ruptured during stent-assisted coil embolization. The M2 branch was occluded after temporary balloon occlusion of the distal M1 and additional coil embolization for bleeding control. The final angiogram revealed nearly complete occlusion of the aneurysm sac and no flow compromise. This patient had severe neurological deficits after embolization and SAH revealed on post-procedural CT. A follow-up CT the next day revealed SAH and severe brain swelling due to cerebral infarction requiring decompressive craniectomy. This patient experienced progressive infarction with hemorrhagic transformation and eventually died after 7 days.
- Procedural coil migration occurred in one patient with a tiny ruptured aneurysm (2 mm in diameter) during a balloon-assisted embolization. The detached coil migrated into the distal M2 branch and on post-procedural angiogram, thrombotic occlusion was seen. After intra-arterial administration of a glycoprotein IIb/IIIa inhibitor (abciximab), acute thrombosis was lysed and the distal MCA flow was improved. At the immediate post-procedural neurological examination, patients did not show neurological differences compared to before the procedure; long-term follow-up DSA showed no flow compromise at the coil migrated distal MCA branch.
- Acute thromboembolic complications occurred in 5 unruptured and 5 ruptured aneurysms in 10 patients. After intra-arterial administration of a glycoprotein IIb/IIIa inhibitor, acute thromboembolism was completely lysed in 9 patients, except for one patient with a ruptured aneurysm. One patient without recanalization after a glycoprotein IIb/IIIa inhibitor injection experienced major cerebral infarction and deterioration of consciousness level after the procedure and finally expired after 3 weeks. One patient with a ruptured aneurysm and recanalization after a glycoprotein IIb/IIIa inhibitor injection presented with progressive infarction within a day and was discharged at mRS2. Two of 5 acute thromboembolic patients with unruptured aneurysm presented with symptomatic acute stroke at 6 and 10 days after treatment even though no neurological deterioration was revealed on immediate post-procedural neurologic examination. The patients were discharged without neurologic deficit. But, the patients visited the emergency room for acute stroke and were discharged at mRS 1 and 4.
- The rate of early post-procedural morbidity and mortality was 6.1% and 3.0% for unruptured aneurysms compared to 2.9% and 2.9% for ruptured aneurysms. As a result, over-all procedure-related permanent morbidity and mortality rates were 4.5% and 3.0% for unruptured and ruptured aneurysms, respectively. These data are summarized in Table 2.
- In the final clinical grading of 33 unruptured aneurysm patients, 2 symptomatic acute stroke patients were discharged at mRS 1 and 4 respectively. One patient improved to mRS 3. None of the surviving patients had any deterioration of functional neurological outcomes (mRS, 0-2).
- Thirty-four patients with ruptured aneurysm were discharged with mRS 0 in 7 patients, mRS 1 in 10 patients, mRS 2 in 3 patients, mRS 3 in 2 patients, mRS 4 in 1 patient, mRS 5 in 4 patients, and mRS 6 in 7 patients. Therefore, there were 20 patients in the favorable outcome group (mRS, 0–2) and 14 patients in the unfavorable outcome group (mRS, 3–6). Seven patients were hospitalized with HHGS IV or V and severe cerebral hemorrhage at the time of visit. They were mostly elderly and expired with severe cerebral hemorrhage and other systemic complications including pneumonia and multiple organ damage.
- Clinical follow-up was available only in 12 of 34 ruptured patients because 22 patients were transferred to another hospital, died or outpatient visits were impossible. Thirty-two aneurysms in 31of the 45 patients who could visit as outpatients at least once at 6–24 months (mean, 26 months; range, 6–96 months) received follow-up angiography. Follow-up angiographic results revealed 1 major and 3 minor recanalizations. One major recanalization aneurysm was re-treated by coiling with no complications.
Results
- Ausman was the first neurosurgeon to advocate EVT of cerebral aneurysms as a first option treatment in 1997. In his experience, 50% of aneurysms were suitable for EVT [14]. Unlike coiling of other cerebral aneurysm locations, the success rate of MCA coiling was initially low at the beginning. In 1999, Regli et al. [2] reported on a consecutive series of 30 patients with 34 unruptured MCA aneurysms. Of the 34 aneurysms evaluated, only 2 (6%) were successfully obliterated with endovascular coil embolization. In 32% of the cases, EVT was attempted but abandoned, and in the remaining 62%, surgery was considered the best therapeutic choice. In this series, 94% of the unruptured MCA aneurysms were treated with surgical clipping. Until recently, EVT for MCA aneurysms showed a higher procedural failure rate and inconsistent results compared with EVT application to aneurysms at other sites [2,6-8,15,16].
- MCA aneurysms have been traditionally considered not ideal for coiling because of their unfavorable anatomic features, including a wide neck and partial incorporation of 1 or both M2 branches [17]. Although the ISAT and some reports [18,19] have confirmed that EVT was equal or superior to clipping for all aneurysms regardless of their location, the treatment of MCA aneurysms, which accounted for 14% of aneurysms, is still controversial [20]. Recently, with the development of interventional devices and technologies, various neck remodeling endovascular techniques have attempted to treat MCA aneurysms with complex morphologic features. Even if EVT technology has advanced recently, neurovascular surgeons still seem to prefer clipping to coiling for MCA aneurysms. Suzuki et al. [15] reported a consecutive series of 115 patients with MCA aneurysms in which only 40% of patients were treated with coiling. Similarly, another recent study of 152 MCA aneurysms reported that 32.6% of the MCA aneurysms were either not considered for EVT or sent to surgery after EVT was attempted [16].
- However, the selection rate for EVT of MCA aneurysms did not appear to be due to the difficulty of treating aneurysms via this technique. It seems that the difference is between medical institutions that consider MCA aneurysm embolization to be the first treatment modality and institutions that still believe surgery is the predominant treatment modality for MCA aneurysms. For example, there are some centers where embolization is the first treatment option considered [7,10]. In contrast, some institutions have applied very stringent criteria to select MCA aneurysm patients who will be treated with EVT. Raftopoulos et al. [4] chose EVT only when the ratio of neck to sac was less than 1:3. Our neurovascular team considered EVT as the first treatment modality for MCA aneurysms. Although almost all MCA aneurysms (95.8%) were treated with neck remodeling techniques, the technical success rate of EVT was 100% in this study. EVT for MCA aneurysms could be a technically feasible treatment strategy.
- One major concern about EVT for MCA aneurysms is procedure-related complications. Choi et al. [21] compared the outcomes of clipping and coiling and evaluated the benefits of clipping for 178 ruptured and unruptured MCA aneurysms. Inclusion criteria for coiling were very strict and only 25 of 178 MCA aneurysms were treated by coiling. In their study, a multidisciplinary neurovascular team decided the treatment strategy (observation, clip, or coil) after discussion. If they expected there would be no definite benefits between the 2 treatment options (clip or coil) in terms of patient outcome and perioperative complications, they primarily chose microsurgical clipping. EVTs were considered in some selected cases that lacked complexity such as a relatively small neck (≤4 mm), large dome-to-neck ratio (>1.5), no incorporated branches in angiographic findings, clinically ruptured aneurysms with a high Hunt-Hess grade and/or use of medications such as warfarin. In the surgical group, perioperative complications occurred in 17 patients (11.1%). However, in the strictly selected EVT group, 8 patients (32%) experienced procedural complications (25% in the ruptured group and 35% in the unruptured group). Therefore, the complication rate for MCA embolization may seem to be high. However, most of the complications included 5 asymptomatic embolic infarctions observed on diffusion magnetic resonance (MR) imaging and 1 asymptomatic intraprocedural aneurysm rupture. Furthermore, there were no cases of procedure-related morbidity and mortality in the EVT group. Their results suggested that EVT for MCA aneurysms in selected patients can be effectively performed but the complication rate is not negligible.
- Generally, complications during EVT were stratified into three groups: (1) thromboembolic complications, (2) parent artery occlusion, and (3) aneurysm perforation [22]. But, it is not easy to analyze EVT complications compared to the complications of surgery. Because complications were determined by the neurointerventionalist who performed the procedure, there are many other factors that can or cannot be classified as complications.
- The first reason is that EVT cases have not been analyzed yet and there are not enough to compare to clipping cases. The next reason is that the definition of EVT complications is vague and unclear. In the case of asymptomatic embolic infarction, institutions which perform MR diffusion after the procedure report it as a complication. But, it is not a complication at institutions that do not perform MR diffusion after the procedure.
- In addition, aneurysm rupture during clipping is not classified as a complication. This is probably because most of them are controllable in the surgical field and do not cause other serious symptoms. Unlike clipping, aneurysm ruptures during embolization may cause serious symptoms and may lead to death or disability. However, many cases of intraprocedural rupture can be treated asymptomatically. Therefore, it is unclear if asymptomatic rupture should be classified as a complication.
- Most of the complications in this study were thromboembolism. We thought at first this was related to antiplatelet premedication. Thromboembolic complications occurred mainly in patients who did not receive premedication. In the early days of attempting unruptured MCA coiling, premedication was not administrated.
- The second cause of thromboembolism was related to selection criteria and the endovascular technique. We performed EVT as a first treatment for MCA aneurysms regardless of their morphological complexity. Most cases (95.8%) could by treated by neck-remodeling techniques using multiple catheters, balloon, or stent. These complex procedures using multiple intravascular devices were known to be a cause of thromboembolic complications [23].
- Lastly, we performed the procedure with the goal of complete occlusion. Therefore, immediate angiographic outcomes revealed complete occlusion in 66 cases (91.7%) by the Raymond classification. Trying complete occlusion of an aneurysm may be likely to increase the incidence of thromboembolic complications during the procedure. Quadros et al. [7] prioritized prevention of thromboembolic complications during MCA aneurysm coiling. They did not treat with a complex, neck-remodeling technique in many cases and left the neck portion of the aneurysms to reduce the rate of thromboembolic complications.
- Fortunately, thromboembolism could be prevented or resolved with administration of antiplatelet premedication or glycoprotein IIb/IIIa inhibitors. In this study, thromboembolism occurred in 10 patients. Although 2 patients experienced immediate post-procedural symptomatic ischemic events, procedural thromboembolism was completely lysed with intra-arterial administration of glycoprotein IIb/IIIa inhibitor. Therefore, like intraprocedural aneurysm rupture, it is unclear that the asymptomatic procedural thromboembolism should be classified as a complication.
- Another major concern is the recurrence rate after coiling for MCA aneurysms [7]. Immediate angiographic complete occlusion was known to an important factor for decreasing recurrence rate after coiling [24]. Brinjikji at al. [22] reported in a review article of 12 series that post-operative complete occlusion was 82.4% and follow-up results of 758 aneurysms demonstrated that 70 (9.3%) had a minor recurrence not requiring re-treatment and 73 (9.6%) had major recanalization requiring retreatment. Overall angiographic stability or progression to better obliteration was reported in 81% of patients undergoing follow-up angiography. However, in our series, immediate angiographic outcomes were 91.7% complete occlusion; only 1 major recanalization (3.1%) was detected on follow-up angiography. We thought that this follow-up angiographic outcome might be related to complete occlusion immediately after the procedure.
- There are some limitations in this study. First, this is a retrospective study and included a small number of MCA aneurysm cases at only 1 institution. Second is patient selection bias. As mentioned above, EVT is a first treatment option for MCA aneurysms in this study regardless of aneurysm morphological complexity. Considering improved operator experience and devices, our result may not represent the general situation of MCA coiling in this era because our cases were collected over 10 years.
- Coil embolization of MCA aneurysms could be a technically feasible and clinically effective treatment strategy with acceptable angiographic and clinical outcomes. In the future, increased operator experience, improved new devices, and various neck-remodeling techniques might greatly increase the feasibility and efficacy of MCA aneurysm coiling.
- However, the safety and efficacy of this technique as compared to surgical clipping remains to be determined.
Discussion
-
Acknowledgements
- This work was supported by a 2-Year Research Grant of Pusan National University.
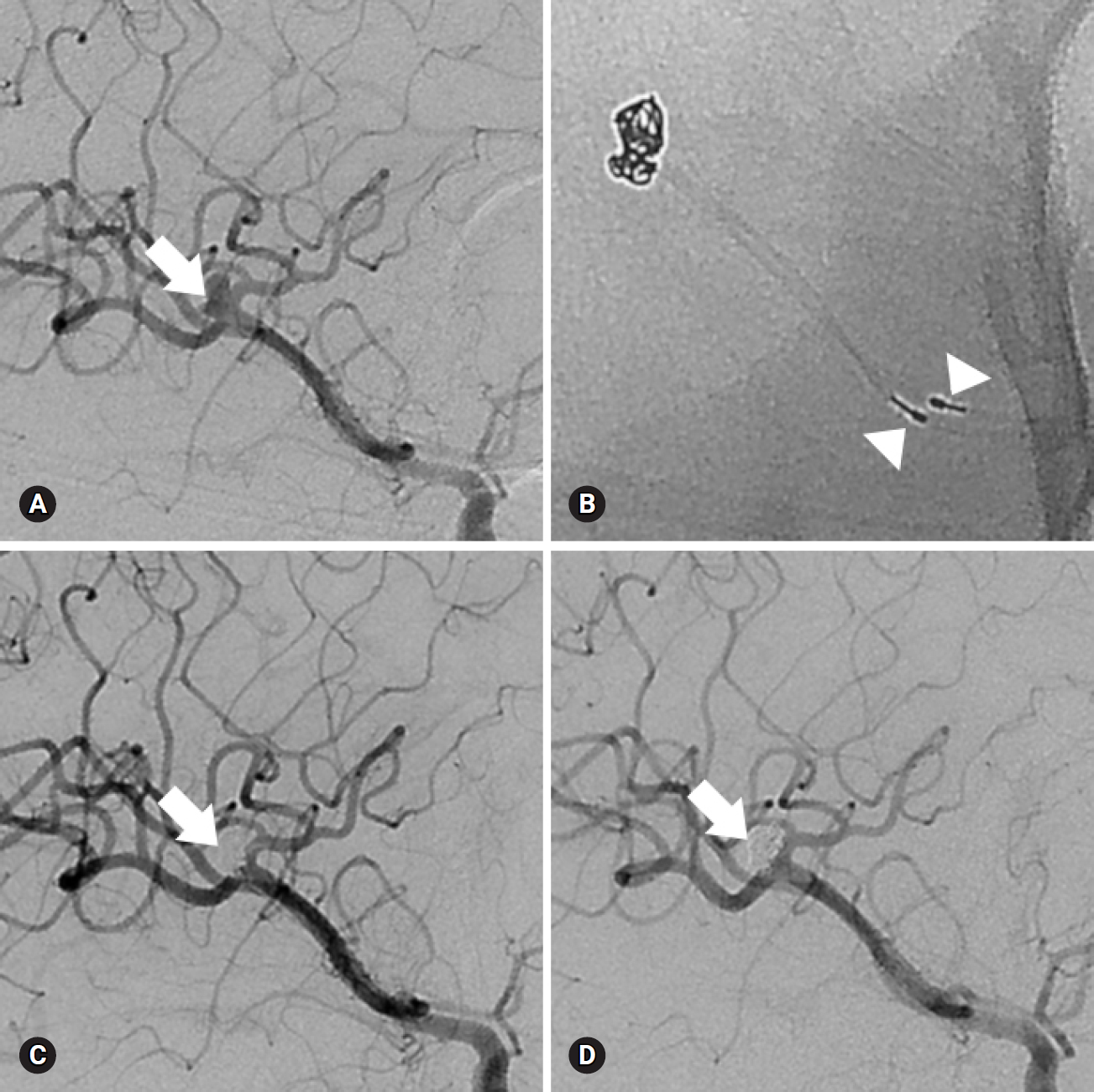
Fig. 1.A 43-year-old man presented with a subarachnoid hemorrhage. (A) Anteroposterior oblique view of left internal carotid angiogram shows a wide-necked aneurysm (arrow) at the right middle cerebral artery bifurcation. (B) The aneurysm is treated with two catheter technique (arrowheads). (C) Final control angiogram reveals complete occlusion of the aneurysm (arrow) without flow compromise of the parent artery. (D) Eighteen-month follow-up angiogram shows stable, complete occlusion of the aneurysm (arrow).


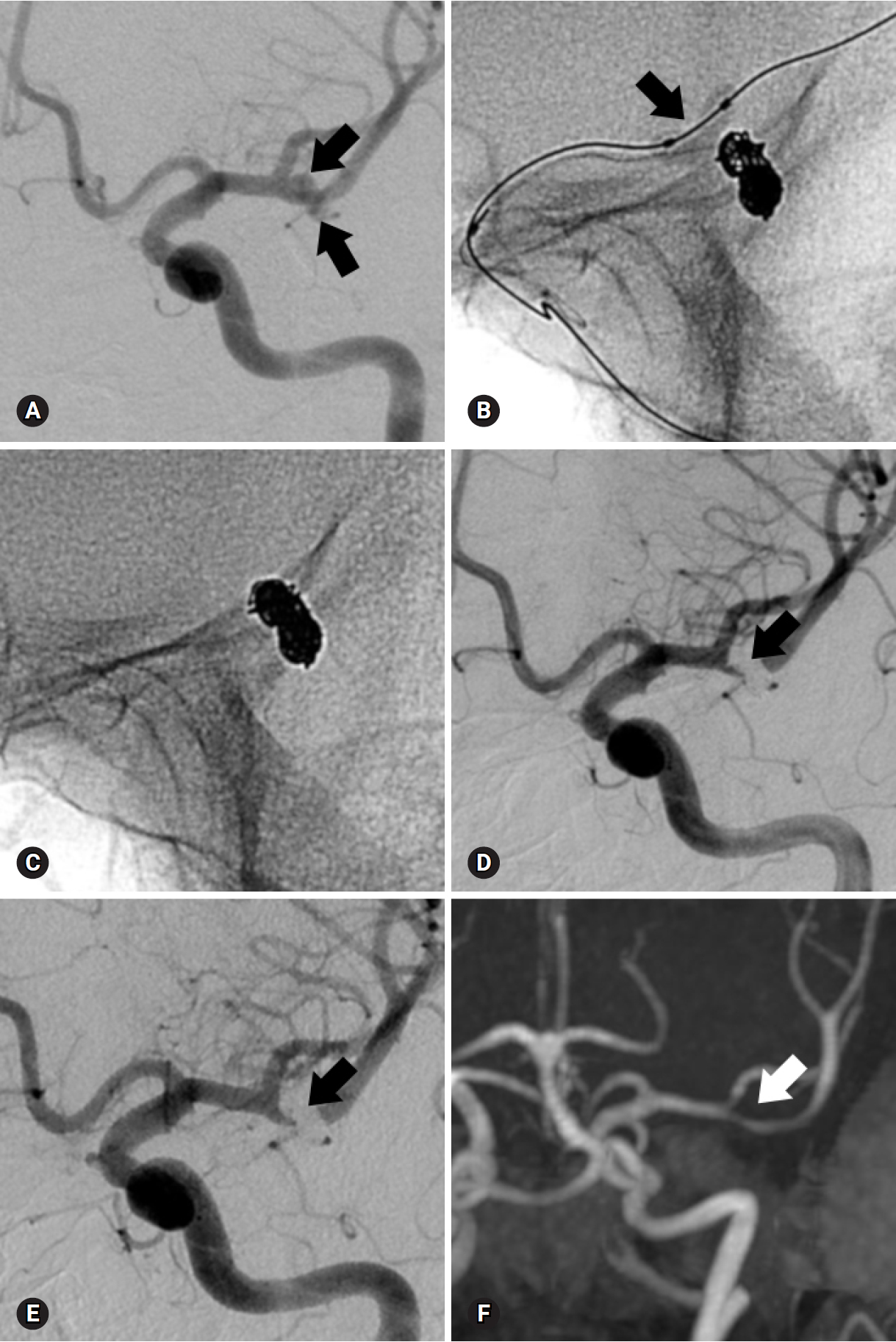
Fig. 2.A 73-year-old woman with a ruptured aneurysm at the left middle cerebral artery bifurcation. (A) Anteroposterior oblique view of left internal carotid angiogram shows an elongated aneurysm (arrows). (B) The aneurysm is treated with balloon-assisted technique (arrow) due to coil protrusion into the parent artery at coil insertion into the neck portion. Immediate post-procedural radiograph (C) and angiogram (D) reveal complete occlusion of the aneurysm (arrow) without coil protrusion into parent artery. Follow-up 18-month angiogram (E) and 41-month magnetic resonance angiogram (F) show stable, complete occlusion of the aneurysm (arrow).
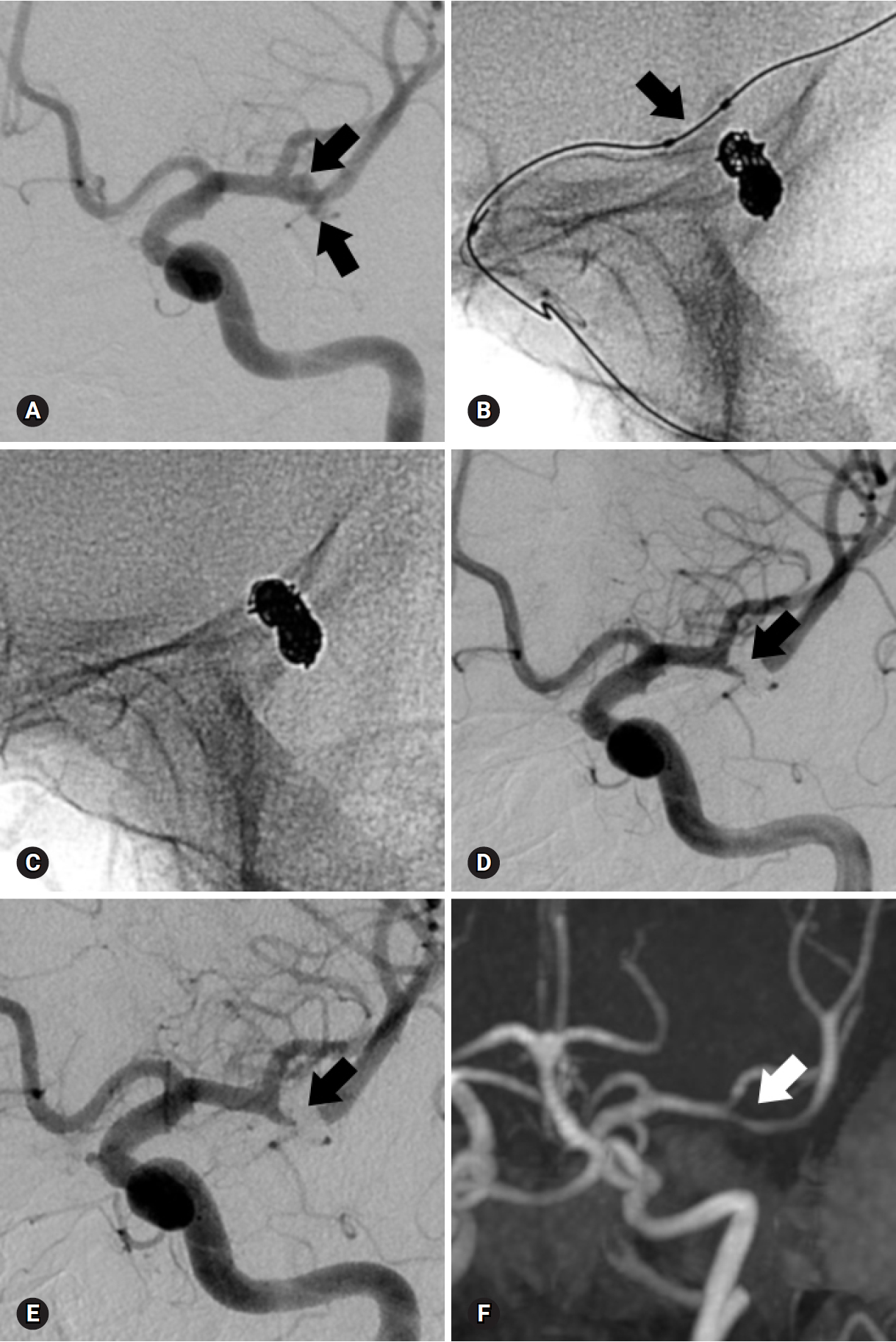

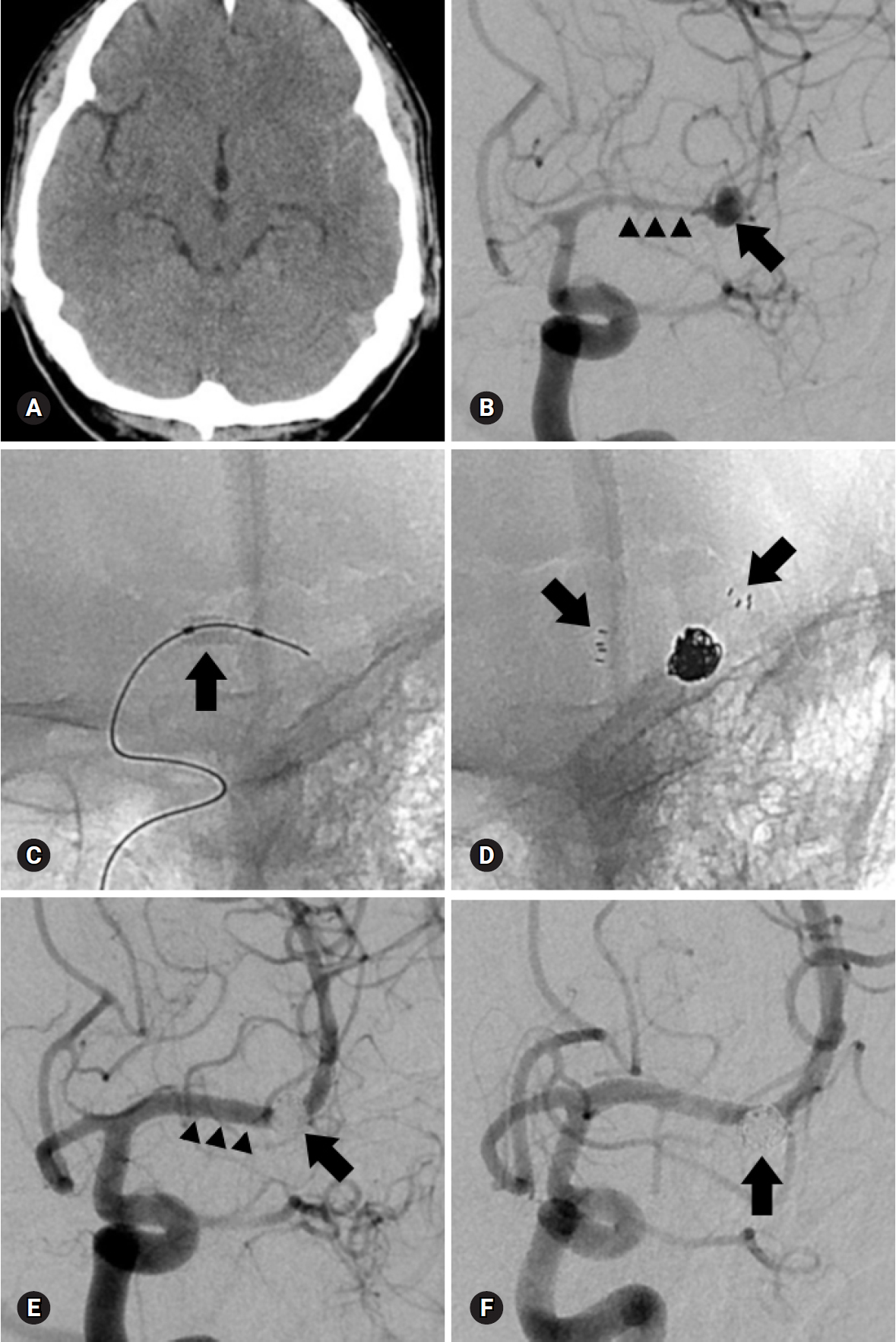
Fig. 3.A 52-year-old man presented with severe headache. (A) Non-enhanced brain computed tomography reveals subarachnoid hemorrhage at the left Sylvian fissure. (B) Anteroposterior oblique view of left internal carotid angiogram shows a wide-necked aneurysm (arrow) at the left middle cerebral artery bifurcation and moderate to severe vasospasm of the anterior and middle cerebral arteries (arrowheads). (C) Prior to coil embolization, angioplasty using a compliant balloon (arrow) is performed to resolve vasospasm. The aneurysm is treated with a stent-assisted technique (arrows) and immediate post-procedural radiograph (D) and angiogram (E) reveal complete occlusion of the aneurysm (arrow) and restoration of vasospasm (arrowheads). (F) A 6-month follow-up angiogram after the procedure demonstrates stable complete occlusion of the aneurysm (arrow) and well-preserved parent artery.
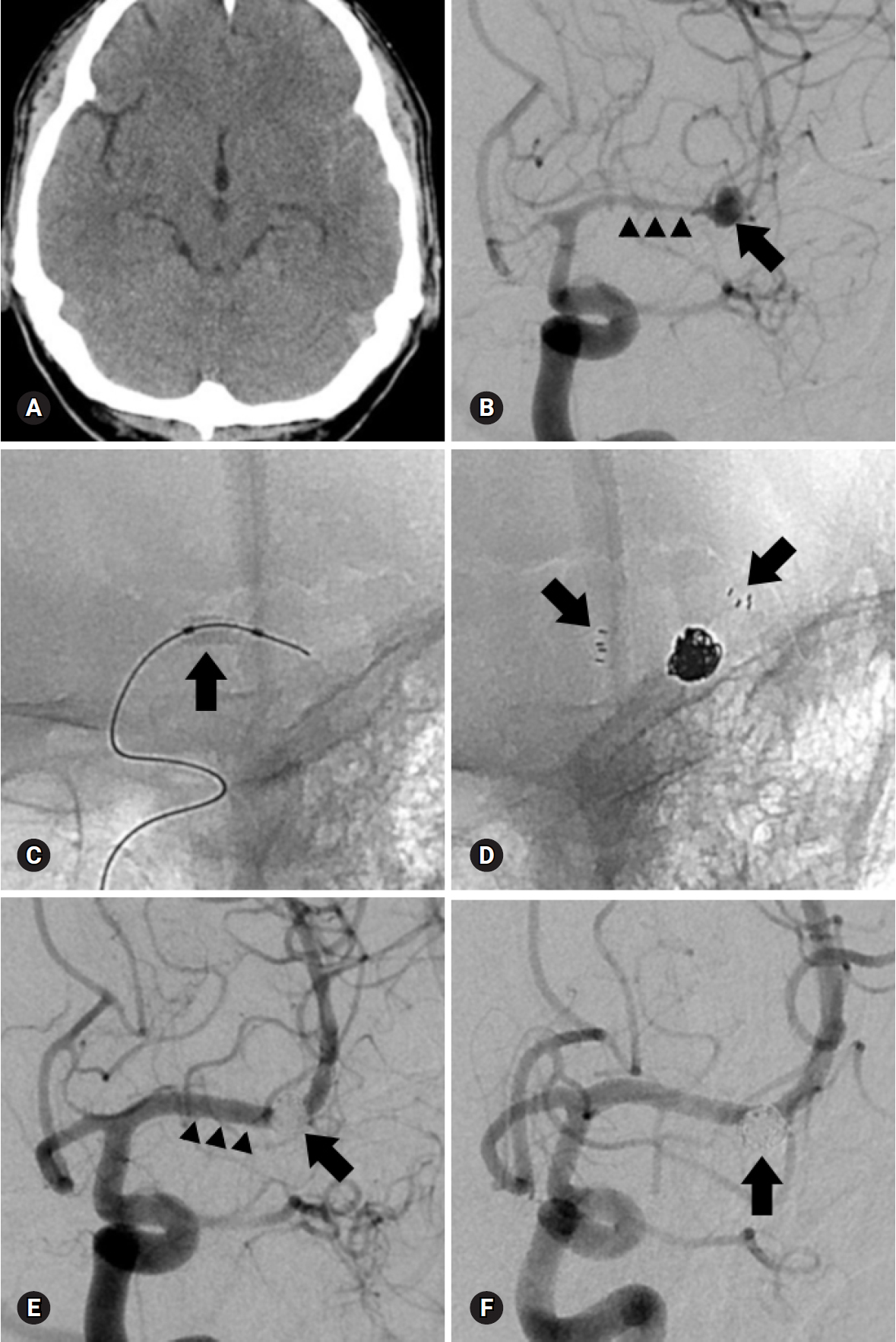

Table 1.Patients and aneurysm characteristics
Table 2.Endovascular treatment, complications, and angiographic and clinical outcomes
- 1. Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002;360:1267–74.ArticlePubMed
- 2. Regli L, Uske A, de Tribolet N. Endovascular coil placement compared with surgical clipping for the treatment of unruptured middle cerebral artery aneurysms: a consecutive series. J Neurosurg 1999;90:1025–30.ArticlePubMed
- 3. Regli L, Dehdashti AR, Uske A, de Tribolet N. Endovascular coiling compared with surgical clipping for the treatment of unruptured middle cerebral artery aneurysms: an update. Acta Neurochir Suppl 2002;82:41–6.ArticlePubMed
- 4. Raftopoulos C, Mathurin P, Boscherini D, Billa RF, van Boven M, Hantson P. Prospective analysis of aneurysm treatment in a series of 103 consecutive patients when endovascular embolization is considered the first option. J Neurosurg 2000;93:175–82.Article
- 5. Iijima A, Piotin M, Mounayer C, Spelle L, Weill A, Moret J. Endovascular treatment with coils of 149 middle cerebral artery berry aneurysms. Radiology 2005;237:611–9.ArticlePubMed
- 6. Doerfler A, Wanke I, Goericke SL, Wiedemayer H, Engelhorn T, Gizewski ER, et al. Endovascular treatment of middle cerebral artery aneurysms with electrolytically detachable coils. AJNR Am J Neuroradiol 2006;27:513–20.PubMedPMC
- 7. Quadros RS, Gallas S, Noudel R, Rousseaux P, Pierot L. Endovascular treatment of middle cerebral artery aneurysms as first option: a single center experience of 92 aneurysms. AJNR Am J Neuroradiol 2007;28:1567–72.ArticlePubMedPMC
- 8. Horowitz M, Gupta R, Gologorsky Y, Jovin T, Genevro J, Levy E, et al. Clinical and anatomic outcomes after endovascular coiling of middle cerebral artery aneurysms: report on 30 treated aneurysms and review of the literature. Surg Neurol 2006;66:167–71.ArticlePubMed
- 9. Kim J, Chang C, Jung Y. Feasibility and midterm outcomes of endovascular coil embolization of an unruptured middle cerebral artery aneurysm with an incorporated branch. World Neurosurg 2018;118:e745–52.ArticlePubMed
- 10. Kim BM, Park SI, Kim DJ, Kim DI, Suh SH, Kwon TH, et al. Endovascular coil embolization of aneurysms with a branch incorporated into the sac. AJNR Am J Neuroradiol 2010;31:145–51.ArticlePubMedPMC
- 11. Lubicz B, Leclerc X, Gauvrit JY, Lejeune JP, Pruvo JP. HyperForm remodeling-balloon for endovascular treatment of wide-neck intracranial aneurysms. AJNR Am J Neuroradiol 2004;25:1381–3.PubMedPMC
- 12. Lubicz B, Lefranc F, Levivier M, Dewitte O, Pirotte B, Brotchi J, et al. Endovascular treatment of intracranial aneurysms with a branch arising from the sac. AJNR Am J Neuroradiol 2006;27:142–7.PubMedPMC
- 13. Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke 2001;32:1998–2004.ArticlePubMed
- 14. Ausman JI. The future of neurovascular surgery. Part I: intracranial aneurysms. Surg Neurol 1997;48:98–100.ArticlePubMed
- 15. Suzuki S, Tateshima S, Jahan R, Duckwiler GR, Murayama Y, Gonzalez NR, et al. Endovascular treatment of middle cerebral artery aneurysms with detachable coils: angiographic and clinical outcomes in 115 consecutive patients. Neurosurgery 2009;64:876–88.ArticlePubMedPDF
- 16. Bracard S, Abdel-Kerim A, Thuillier L, Klein O, Anxionnat R, Finitsis S, et al. Endovascular coil occlusion of 152 middle cerebral artery aneurysms: initial and midterm angiographic and clinical results. J Neurosurg 2010;112:703–8.ArticlePubMed
- 17. Jayaraman MV, Do HM, Versnick EJ, Steinberg GK, Marks MP. Morphologic assessment of middle cerebral artery aneurysms for endovascular treatment. J Stroke Cerebrovasc Dis 2007;16:52–6.ArticlePubMed
- 18. Higashida RT, Lahue BJ, Torbey MT, Hopkins LN, Leip E, Hanley DF. Treatment of unruptured intracranial aneurysms: a nationwide assessment of effectiveness. AJNR Am J Neuroradiol 2007;28:146–51.PubMedPMC
- 19. van Rooij WJ, Sluzewski M. Procedural morbidity and mortality of elective coil treatment of unruptured intracranial aneurysms. AJNR Am J Neuroradiol 2006;27:1678–80.PubMedPMC
- 20. Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005;366:809–17.ArticlePubMed
- 21. Choi JH, Park JE, Kim MJ, Kim BS, Shin YS. Aneurysmal neck clipping as the primary treatment option for both ruptured and unruptured middle cerebral artery aneurysms. J Korean Neurosurg Soc 2016;59:269–75.ArticlePubMedPMCPDF
- 22. Brinjikji W, Lanzino G, Cloft HJ, Rabinstein A, Kallmes DF. Endovascular treatment of middle cerebral artery aneurysms: a systematic review and single-center series. Neurosurgery 2011;68:397–402.ArticlePubMedPDF
- 23. Kim SR, Vora N, Jovin TG, Gupta R, Thomas A, Kassam A, et al. Anatomic results and complications of stent-assisted coil embolization of intracranial aneurysms. Interv Neuroradiol 2008;14:267–84.ArticlePubMedPMC
- 24. Nishido H, Piotin M, Bartolini B, Pistocchi S, Redjem H, Blanc R. Analysis of complications and recurrences of aneurysm coiling with special emphasis on the stent-assisted technique. AJNR Am J Neuroradiol 2014;35:339–44.ArticlePubMedPMC
References
Figure & Data
References
Citations
Citations to this article as recorded by 

- Adverse events during endovascular treatment of ruptured aneurysms: A prospective nationwide study on subarachnoid hemorrhage in Sweden
Bryndís Baldvinsdóttir, Paula Klurfan, Johanna Eneling, Elisabeth Ronne-Engström, Per Enblad, Peter Lindvall, Helena Aineskog, Steen Friðriksson, Mikael Svensson, Peter Alpkvist, Jan Hillman, Erik Kronvall, Ola G. Nilsson
Brain and Spine.2023; 3: 102708. CrossRef - Microsurgical Clipping versus Advanced Endovascular Treatment of Unruptured Middle Cerebral Artery Bifurcation Aneurysms After a “Coil-First” Policy
Muriel Pflaeging, Christoph Kabbasch, Marc Schlamann, Lenhard Pennig, Stephanie Theresa Juenger, Jan-Peter Grunz, Marco Timmer, Gerrit Brinker, Roland Goldbrunner, Boris Krischek, Lukas Goertz
World Neurosurgery.2021; 149: e336. CrossRef

 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine PubReader
PubReader ePub Link
ePub Link Cite
Cite