Intravesical bacillus Calmette–Guérin-induced myopathy presenting as rhabdomyolysis: a case report
Article information
Abstract
Intravesical bacillus Calmette–Guérin (BCG) instillation is an adjuvant treatment for non–muscle-invasive urinary bladder cancer. Although most complications associated with BCG immunotherapy are mild and self-limiting, rare albeit serious complications have been reported. Only a few cases of BCG-related rhabdomyolysis have been reported. In this study, we present the case of a 72-year-old woman who developed severe weakness and hyperCKemia following intravesical BCG instillation. A muscle biopsy was performed, and a diagnosis of drug-induced myopathy was made.
Introduction
Intravesical bacillus Calmette–Géurin (BCG) instillation is an effective therapy for superficial bladder carcinoma. The BCG vaccine is a live attenuated form of Mycobacterium bovis that triggers a variety of local immune responses, eliciting antitumor activity. Although usually considered safe, various BCG-related adverse events have been reported, including disseminated BCG infection, fever, arthritis, uveitis, hepatitis, and genitourinary infection [1]. Rhabdomyolysis is a rare but potentially life-threatening skeletomuscular complication associated with intravesical BCG instillation. However, very little is known about the clinical progress and histological findings of rhabdomyolysis associated with BCG immunotherapy. Here, we report the case of a 72-year-old woman who developed subacute-onset motor weakness after weekly induction therapy with intravesical BCG instillation.
Case
Ethical statements: This study was approved by the Institutional Review Board (IRB) of Severance Hospital (IRB No: 4-2022-1139), and the requirement for informed consent from the patient was waived by the IRB.
A 72-year-old woman was referred to the department of neurology for progressive weakness. She was being administered rosuvastatin 20 mg to manage dyslipidemia. She had a history of rectal and endometrial cancers, both of which were treated without evidence of recurrence. The patient had also been diagnosed with an early mucosal urinary bladder cancer and had undergone transurethral resection of the tumor 2 months prior to the consultation. Weekly intravesical BCG instillation therapy was initiated after tumor resection (Fig. 1). Eight days after the third BCG instillation, the patient experienced a sudden onset of weakness in her legs. During the following weeks, the patient gradually had trouble getting up from a chair, washing her face, and maintaining a standing position. She was readmitted 3 weeks after her onset of weakness, at which time she was completely bedridden. Her vital signs at admission were stable: blood pressure, 121/59 mmHg; heart rate, 77 beats/min; respiratory rate, 16 breaths/min; and body temperature, 37.3°C. Neurological examination revealed symmetrical proximal muscle weakness in the arms (modified Medical Research Council [MRC] grade 2) and in the legs (modified MRC grade 3). Deep tendon reflexes were normoactive, and a sensory examination showed normal results. She denied symptoms of myalgia or urine color change. The results of blood tests were as follows: hemoglobin, 6.7 g/dL (range, 11.4–16.0 g/dL); white blood cell count, 12,850/μL (range, 4,000–10,800/μL); neutrophils, 89.1%; platelet count 265,000/μL (range, 150,000–400,000/μL); aspartate aminotransferase, 2,235 IU/L (range, 13–34 IU/L); alanine aminotransferase, 1,189 IU/L (range, 5–46 IU/L); blood urea nitrogen, 146.4 mg/dL (range, 7.3–20.5 mg/dL); creatinine, 5.74 mg/dL (range, 0.49–0.91 mg/dL; sodium, 135 mmol/L (range, 135–145 mmol/L); potassium, 8.0 mmol/L (range, 3.5–5.5 mmol/L); and chloride, 110 mmol/L (range, 98–110 mmol/L). Her serum creatinine kinase (CK) level was markedly increased (>13,000 IU/L). The patient underwent further evaluations to reveal the cause of her weakness. A nerve conduction study revealed normal results. Electromyography indicated short-duration and small-amplitude motor unit action potentials in the arms and legs, but abnormal spontaneous activities were not observed. Muscle magnetic resonance imaging revealed diffuse T2 high signal intensity in the whole-body muscles with subcutaneous fat infiltration (Fig. 2). Rhabdomyolysis with acute kidney injury was diagnosed, and hemodialysis was initiated. However, the patient’s serum CK level remained >13,000 IU/L even weeks after admission. Muscle biopsy was conducted at the vastus lateralis muscle for differential diagnosis, which showed increased variability in fiber size, muscle fiber splitting, and type 1 fiber atrophy with mild lymphocytic infiltration. Faint sarcolemmal staining of the major histocompatibility complex (MHC)-1 and membrane attack complex (C5b–C9) in non-necrotic fibers was observed (Fig. 3). Diagnosis of drug-induced myopathy was made, and conservative management was maintained without immunotherapy. One month after admission, her leg weakness improved to a modified MRC grade of 4, and her serum CK level decreased to 622 IU/L. The patient was able to walk without assistance 2 months after admission, at which time her serum CK level was normal.
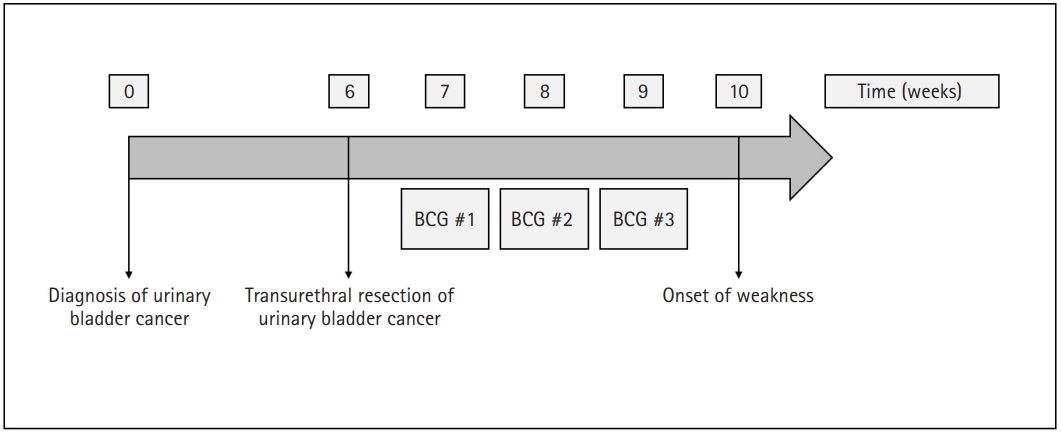
Time course of the treatment for urinary bladder cancer and onset of symptoms in weeks. BCG, bacillus Calmette–Guérin.
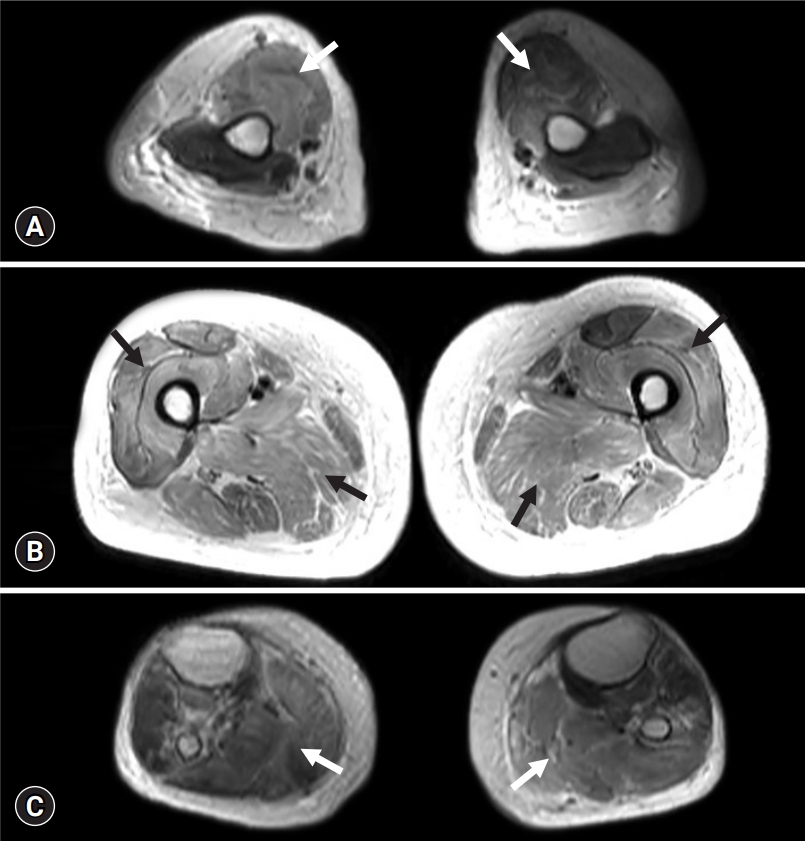
Whole-body muscle magnetic resonance imaging of the present case. Axial T2-weighted modified Dixon images show symmetric bilateral hyperintense signal of the muscle (arrows) in the (A) upper arm, (B) thigh, and (C) lower leg.

Immunohistochemistry of the muscle biopsy. (A) Hematoxylin and eosin stain (×400) shows increased size variability and degeneration with mild lymphocytic infiltration. (B) Myosin ATPase stain (×100) at pH 4.3 shows type 1 fiber atrophy. In immunohistochemical stain of sarcolemma, (C) major histocompatibility complex-1 (×100) and (D) C5b–C9 (×100) are faintly expressed.
Discussion
Herein, we report a case of drug-induced myopathy following intravesical BCG instillation. The patient experienced rapidly increasing weakness and became completely bedridden. The serum CK level was markedly elevated, and muscle biopsy showed mild lymphocytic infiltration. These findings were suggestive of toxic myopathy or immune-mediated necrotizing myopathy (IMNM). However, in contrast to the known clinical course of IMNM, which is often chronic and less responsive to treatment, there was full recovery with only conservative management in the present case. Although the serostatus of the autoantibodies associated with IMNM was not determined in the present case, we believed that the weakness was caused by the direct toxic effect of medication rather than the induction of inflammatory processes as is seen in IMNM. In addition, based on the temporal relationship between the date of medication use and onset of symptoms, we considered that BCG was more likely to be the cause of myopathy than rosuvastatin. Musculoskeletal adverse events usually occur within 1 month following the administration of a statin [2,3]; therefore, it was less likely for rosuvastatin, which had been used for more than 2 years, to have caused myopathy in the present case. Based on these assumptions, a diagnosis of drug-induced myopathy associated with BCG instillation was made.
Only a few reports of rhabdomyolysis after BCG instillation have been published. Armstrong [4] reported a 64-year-old man who developed severe myalgia and had an elevated CK level (>60,000 U/mL) 6 hours after BCG instillation. Despite intensive hydration, his limb weakness, difficulty in swallowing, and respiratory muscle weakness progressed. The patient became anuric, and hemodialysis was initiated. Approximately 1 month after admission, spontaneous diuresis ensued, and the patient was able to walk with assistance. Another report described a 72-year-old man who presented with fever and weakness 4 hours after BCG instillation. Disseminated intravascular coagulopathy, intravascular hemolysis, and diuretic-resistant oliguria progressed, and the patient died of multi-organ dysfunction 17 days after the first symptoms appeared [5]. As in previous cases, the patient in the present case showed acute onset of weakness, highly elevated serum CK levels, and acute renal failure.
Histological findings of myopathy associated with BCG instillation have not been previously elucidated. Drug-induced myopathies can present in various forms, including necrotizing myopathy, inflammatory myopathy, type 2 fiber atrophy, mitochondrial myopathy, and myofibrillar myopathy [6]. The histological findings in the present case showed inflammatory cell infiltration and type 1 fiber degeneration without prominent upregulation of MHC-1. These findings are similar to those of necrotizing myopathy, which is associated with lipid-lowering drugs in terms of scattered necrotic fibers, less prominent lymphocytic infiltration, and the absence of MHC-1 upregulation [7]. However, in contrast to the present results, myopathy associated with lipid-lowering agents is frequently associated with type 2 fiber atrophy. Type 1 fibers, which were mostly involved in the present case, are reported to be preferentially affected in myopathies associated with chloroquine or colchicine.
Little is known about the underlying mechanism by which BCG instillation causes complications. Most systemic complications associated with intravesical BCG instillation are thought to result from hypersensitivity reactions or disseminated infection by M. bovis [8]. However, it remains unknown whether the same mechanism underlies the myopathy after BCG instillation. The histological findings of the present case and the recovery of weakness without immunotherapy suggest a direct toxic effect of BCG on muscle fibers rather than an indirect effect by activating an immunological response. The mechanisms underlying this case are difficult to elucidate, and further research is needed to investigate the mechanisms of BCG-associated myopathies.
In conclusion, we report a case of rhabdomyolysis after intravesical BCG instillation. Although rare, practitioners should be aware of this potentially critical complication and be able to explain it to patients before the procedure.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (2019R1C1C1009875).
Author contributions
Conceptualization: all authors; Data curation: CHL, BJC, JHK, TWY; Formal analysis: CHL, GHK, HYS, SHK, SWK; Investigation: CHL, BJC, JHK, TWY, GHK; Funding acquisition: SWK; Supervision: HYS, SHK, SWK; Writing-original draft: CHL, BJC; Writing-review & editing: JHK, TWY, GHK, HYS, SHK, SWK.
