Infection prevention measures and outcomes for surgical patients during a COVID-19 outbreak in a tertiary hospital in Daegu, South Korea: a retrospective observational study
Article information
Abstract
Background
The first large coronavirus disease 2019 (COVID–19) outbreak outside China occurred in Daegu. In response, we developed infection prevention measures for surgical patients during the outbreak at our hospital and retrospectively reviewed the outcomes of COVID–19–related surgical patients.
Methods
We reviewed the medical records of 118 COVID–19–related surgical patients and monitored their clinical outcomes until March 31, 2021. We also interviewed healthcare workers who participated in their perioperative care at Kyungpook National University Chilgok Hospital. The perioperative management guidelines for COVID–19–related patients were prepared through multidisciplinary discussions, including the infection control department, surgical departments, and anesthesiology department before and during the COVID–19 outbreak.
Results
One standard operating room was temporarily converted to a negative-pressure room by increasing the exhaust air volume, creating a relative pressure of −11.3 Pa. The healthcare workers were equipped with personal protective equipment according to the patient's classification of the risk of COVID–19 transmission. The 118 COVID–19–related patients underwent emergent surgery in the negative–pressure room, including three COVID–19–confirmed patients and five COVID–19–exposed patients.
Conclusion
All surgeries of the COVID–19–related patients were performed without specific adverse events or perioperative COVID–19 transmission. Our experience setting up a negative–pressure operating room and conservative perioperative protocol to prevent COVID–19 transmission will help plan and execute infection control measures in the future.
Introduction
The first case of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in South Korea was confirmed on January 20, 2020, and the first case in Daegu was confirmed on February 18, 2020. The famous 31st case in South Korea was a 61-year-old woman who lived in Daegu and infected more than 1,100 people [1]. After the first confirmed SARS-CoV-2 infection case in Daegu, the cases of laboratory-confirmed SARS-CoV-2 infection increased rapidly to a maximum of 741 patients per day [2]. Daegu became the first city with a large-scale coronavirus disease 2019 (COVID-19) outbreak outside China. During the COVID-19 outbreak in Daegu, 11,037 cases were confirmed, and 222 patients died [2].
Few hospitals in Daegu were prepared to provide perioperative care for COVID-19–related patients before the COVID-19 outbreak. However, perioperative infection control in COVID-19–related patients is crucial in preventing nosocomial infections and maintaining essential hospital services during the COVID-19 outbreak. Therefore, we developed and applied COVID-19 infection control protocols for surgical patients. This study describes our experience delivering perioperative infection prevention and control measures for COVID-19–related surgical patients and retrospectively reviews their clinical outcomes.
Materials and methods
This study was based on Kyungpook National University Chilgok Hospital records and interviews with frontline healthcare workers (HCWs) involved in providing care during the outbreak. A nasopharyngeal swab or sputum sample was collected for SARS-CoV-2 quantitative reverse transcription-polymerase chain reaction (RT-PCR) from the patients involved. SARS-CoV-2 RT-PCR was performed using the PowerChek 2019-nCoV Real-Time PCR kit (Kogene Biotech, Seoul, Korea) and CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA).
The perioperative management guidelines for COVID-19–related patients were prepared through multidisciplinary discussions, including the infection control department, surgical departments, and anesthesiology department before and during the COVID-19 outbreak. The electronic medical records of the COVID-19–related patients who underwent surgery were reviewed, and the clinical outcomes of the patients were monitored up to March 31, 2021, when all the patients had been discharged. We also reviewed the records on remodeling of the positive-pressure operating room (OR) to create the negative-pressure OR.
Results
1. Development of COVID-19 infection control protocols for surgical patients
Before and during the COVID-19 outbreak in Daegu, we developed COVID-19 infection control measures for surgical patients, modified from the infection control protocols for severe acute respiratory syndrome [3] and perioperative considerations for COVID-19 developed by the Anesthesia Patients Safety Foundation [4].
The strategy for perioperative COVID-19 infection control targeted three key areas: (1) classification of patients based on the risk of COVID-19 transmission, (2) protection of HCWs, and (3) reorganization of the OR.
1) Classification of surgical patients based on risk of COVID-19 transmission
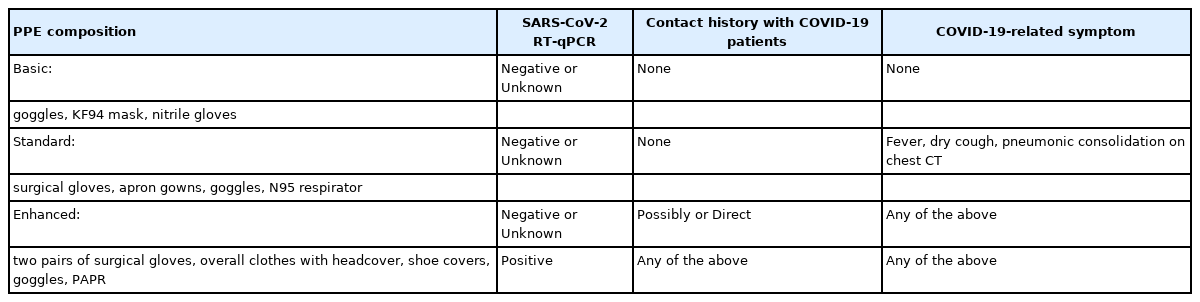
We tested all surgical patients before surgery using SARS-CoV-2 RT-PCR to identify asymptomatic COVID-19 carriers. All surgical patients wore face masks (dental or KF-94, masks able to filter at least 94% of airborne particles) to prevent SARS-CoV-2 transmission. Elective surgeries for COVID-19–confirmed patients were delayed as much as possible. For COVID-19–exposed patients, the surgeries were delayed until after the 14-day incubation period [5]. For emergent surgeries, we classified the surgical patients into four groups: (1) patients who had a negative SARS-CoV-2 RT-PCR test without suspected symptoms (fever, fatigue, dry cough, dyspnea, or pulmonary consolidations on chest computed tomography [CT]) and who did not have a contact history with COVID-19 patients (surgeries were performed in a common OR for this group of patients); (2) patients who had not received their SARS-CoV-2 RT-PCR results before entering the OR or had ambiguous test results, but did not have suspected symptoms or contact history with COVID-19 patients; (3) patients who had suspected symptoms or were exposed to someone with COVID-19, but had negative test results or did not have their SARS-CoV-2 RT-PCR test results before entering the OR; and (4) patients who had a positive SARS-CoV-2 RT-PCR test (Table 1). We classified the last three groups as COVID-19–related surgical patients.
2) Healthcare worker protection
Temperature monitoring was mandatory for all HCWs in the hospital. HCWs with a temperature above 37.5°C, respiratory symptoms, or possible contact history with a confirmed COVID-19 patient without wearing personal protective equipment (PPE) were removed from duty and sent for SARS-CoV-2 RT-PCR testing. In addition, all contact histories between HCWs and COVID-19–related patients were recorded within the OR complex.
PPE was introduced for all HCWs who usually had contact with patients in the hospital. Basic PPE, which consisted of a KF94 mask (mask able to filter at least 94% of airborne particles), eye protection goggles, and latex gloves, was provided to all HCWs in the OR. Standard PPE, which comprised an N95 respirator, surgical cap, eye protection (goggles or face shield), apron gown, and gloves, was provided to HCWs who cared for SARS-CoV-2-negative patients or patients who did not have confirmed test results with COVID-19–related symptoms. Enhanced PPE, which consisted of overall clothes with a headcover, shoe covers, goggles, surgical gloves, and a powered air-purifying respirator (PAPR), was provided to HCWs who managed SARS-CoV-2-confirmed patients, or SARS-CoV-2-exposed patients.
3) Reorganization of the operating room
We reduced elective surgeries to half capacity to limit traffic in the OR during the first 2 months after the outbreak. Surgical procedures for confirmed COVID-19 patients in the intensive care unit (ICU) were performed at the bedside, where possible. We operated on COVID-19–related surgical patients in the designated COVID-19 OR, which had been converted to a negative-pressure OR. When possible, surgeries for confirmed COVID-19 patients and SARS-CoV-2-exposed patients were performed in the last order of the day to minimize contact with other HCWs in the OR complex. These patients were transferred directly to the COVID-19 OR via dedicated paths and an elevator using a portable patient isolation unit with negative pressure (Fig. 1).
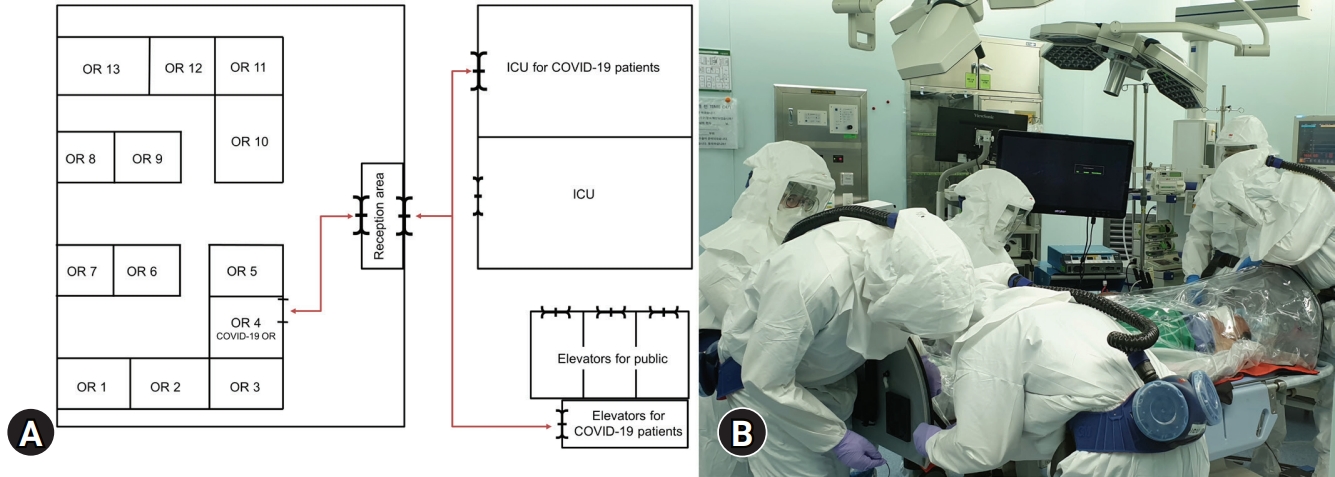
(A) Transfer route for coronavirus disease 2019 (COVID–19)-related patients. The red arrows show all pathways that were closed during COVID–19-related patient transfer. (B) Patient transfer using a portable isolation unit. All healthcare workers wore enhanced personal protective equipment, including overall clothes with headcover, shoe cover, goggles, surgical gloves, and powered air-purifying respirator. OR, operating room; ICU, intensive care unit.
After the COVID-19–related patients entered the COVID-19 OR, anesthesiologists induced anesthesia with endotracheal intubation using the rapid sequence technique. Thirty minutes of ventilation on room air was allowed after endotracheal extubation. The patients recovered from anesthesia in the COVID-19 OR without using the post-anesthesia care unit. After the patients had fully recovered, they were sent back to the ward or ICU using a portable patient isolation unit in negative-pressure mode.
2. Setting up the negative-pressure operating room
Our hospital has 13 ORs in the main operating complex, which is a positive-pressure environment. The rooms allowed 30 air changes per hour and were managed as class 100,000 cleanrooms (less than 100,000 particles/m3). Therefore, we needed to establish a negative-pressure OR to minimize the flow of contaminated air spreading outside the OR [6]. We selected OR number 4 as the COVID-19 OR in the main operating complex because it was easy to access from outside the operating complex and had a transparent glass window.
Outside air was supplied from an inlet duct, and the air in the OR was discharged using a constant air volume unit through the outlet duct. We added a new exhaust duct with a new constant air volume unit to achieve negative pressure (Fig. 2). After this modification, the outflow air volume of OR 4 changed from 450 cubic meters per hour (CMH) to 3,380 CMH, and the room pressure was maintained at −11.3 Pa when the door was closed (below the negative-pressure room standard of −2.5 Pa [7]). Thus, during operation of the negative-pressure OR, exhaust to the clean corridor was limited.
3. Room preparation before and after surgery
Computers, telephones, monitors, and ventilators were draped with plastic film. Anesthetic drugs, fluids, and equipment needed to perform the surgery were prepared before patient arrival. Additionally, we used disposable equipment such as a laryngoscope, surgical drape, and anesthetic circuit. We installed three high-efficiency particulate air (HEPA) filters in the anesthetic circuit (the anesthesia machine’s inspiratory and expiratory limbs and the patient’s side that connects to the endotracheal tube). HCWs in the COVID-19 OR wore dedicated PPE before the COVID-19–related patient entered the OR. After the patient was removed, the room was ventilated for 30 minutes. Subsequently, the HCWs removed and disposed of their PPE in the COVID-19 OR. Surface disinfection was performed using 0.1% sodium hypochlorite. For SARS-CoV-2-confirmed cases, surface disinfection was performed twice.
4. Surgeries for COVID-19–related patients
Between February 22, 2020 and March 31, 2021, 118 COVID-19–related patients underwent emergency surgery. All patients underwent SARS-CoV-2 quantitative RT-PCR testing before surgery. A total of 102 patients who were unable to wait for the test results due to emergency surgery were included in the COVID-19–related patient group. Eight patients were negative on the preoperative test but had a COVID-19–related symptom. Six patients had fevers. Two patients had pneumonic consolidation on the preoperative CT scan. Four patients had a history of direct exposure. Two patients had a prior positive COVID-19 test but were negative at the time of surgery. Two patients were confirmed to be positive for COVID-19 upon preoperative examination.
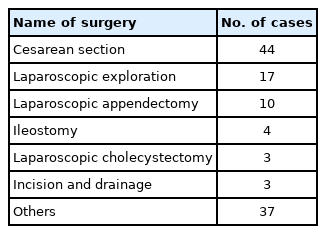
One patient, who was negative based on the preoperative SARS-CoV-2 quantitative RT-PCR result using a nasopharyngeal swab sample, tested positive postoperatively with a sputum sample. The patient had a history of direct exposure to COVID-19 and pneumonic consolidation on preoperative chest CT. Hence, we performed surgery with enhanced PPE. The types of surgeries that the patients underwent are shown in Table 2.
All surgeries for the COVID-19–related patients were uneventful, and perioperative COVID-19 transmission was not reported during this period. We performed 75 cases of general anesthesia and 43 cases of regional anesthesia.
Discussion
SARS-CoV-2 is highly contagious and spreads rapidly. In Hubei province, China, the estimated reproduction number (R) was 4.02, but the R in Daegu, Korea was between 3.472 and 3.543 [8]. An increase in COVID-19 patients in Daegu, Korea was mainly a result of exposure among members of the Shincheonji Church of Jesus and hospital transmission [1].
The COVID-19 outbreak has underscored the importance of preventing nosocomial transmission [9]. HCWs need to be wary of COVID-19 nosocomial transmission, and hospitals should establish and maintain infection control and prevention protocols. We provided a facemask for all surgical patients. In addition, we reduced the number of elective surgeries by half to reduce traffic in the hospital and increased distancing between surgical patients from February 22, 2020 to March 31, 2020.
Approximately 30% to 40% of patients who tested positive for SARS-CoV-2 by quantitative RT-PCR were asymptomatic [10,11]. Therefore, to prevent asymptomatic carrier transmission, we screened all surgical patients for SARS-CoV-2. Sputum or nasopharyngeal swab samples were collected, but sputum samples were preferred if available [12].
The reported sensitivity of SARS-CoV-2 quantitative RT-PCR is approximately 70% to 90% [13]. Furthermore, approximately 3% to 12% of infected patients do not show any suspected symptoms and are reported to be negative. Therefore, we conservatively enrolled COVID-19–related patients and classified them according to contact history and COVID-19–associated symptoms.
SARS-CoV-2 is transmitted through respiratory droplets, contact, fomites, and fecal-oral routes [14,15]. The viral load in the upper respiratory tract is high, and the virus is likely to shed and spread under asymptomatic conditions [16,17]. In addition, SARS-CoV-2 can be transmitted via aerosols [18]. Therefore, we preferred regional anesthesia techniques to minimize aerosol production in the operating theater. In particular, we performed cesarean sections under regional anesthesia in 36 out of 44 cases. We also installed HEPA filters between the anesthetic machine and the patient to decrease the risk of environmental contamination during general anesthesia.
To prevent aerosol transmission, we avoided aerosol-producing interventions such as face mask ventilation and open airway suctioning as much as possible in all anesthesia cases [4,18]. We adopted a rapid sequence intubation technique to prevent aerosol generation during mask ventilation. Intubation and extubation can also generate aerosols. Tracheal extubation produces 15 times more aerosols than intubation [19]. However, aerosol generation during extubation is still less than that of a single cough, and most aerosols generated during extubation may be produced by coughing after extubation [19]. Therefore, these procedures are not included in high-risk aerosol-producing procedures, and cough prevention during induction of anesthesia and after extubation is essential to prevent aerosol generation [20].
PAPR offers full face and respiratory protection. It does not require a fit test for loose fitting [21]. It also provides a cool and comfortable environment for the operator, decreases fatigue, and allows the operator to focus on the procedure. However, there is no strong evidence that it reduces viral transmission more than N95 respirators [22]. In addition, the supply of PAPRs was limited during the early outbreak, and this equipment requires a considerable amount of time to be cleaned and recharged after use. Therefore, we only included PAPR in the enhanced PPE.
We converted a positive-pressure OR to a temporary, negative-pressure OR with an adequate pressure gradient. The negative-pressure OR maintained a continuous negative-pressure gradient (−11.3 Pa) by increasing the exhaust air volume. We did not prepare an anteroom because we did not have a connected OR and did not have time to implement structural changes to the OR complex. Therefore, our HCWs wore and removed their PPE in the COVID-19 OR. We used disposable anesthetic and surgical equipment whenever possible to decrease the risk of virus exposure to sterile processing technicians. The disposed PPE and surgical equipment were sealed in zipper bags.
This study had some limitations. First, we performed only three surgeries in SARS-CoV-2-infected patients who were actively shedding virus because procedures in SARS-CoV-2-infected patients (such as tracheostomy and venoarterial extracorporeal membrane oxygenation cannulae insertion and removal) were typically performed in the ICU. Since there were few surgeries for confirmed patients, there is a limit to establishing the effectiveness of our infection prevention measures. Second, the negative-pressure OR was used mainly for abdominal surgeries. We did not perform neurosurgical or cardiovascular emergency procedures in COVID-19–related patients during this period. However, we believe that this fact does not diminish our infection prevention measures. Third, during PPE shortages, our infection prevention protocols should be conservatively applied. Ten additional SARS-CoV-2-positive patients were confirmed in the hospital during this period, including private caregivers and shop employees. Nevertheless, there were no verified nosocomial transmissions due to the strict adherence to the protocols and guidelines of our conservative infection prevention protocols.
COVID-19 infections and transmission in the hospital setting can lead to breakdown of medical systems. To prevent nosocomial transmission during surgical procedures, we converted a positive-pressure OR to a negative-pressure OR and implemented a perioperative protocol to prevent COVID-19 transmission to HCWs. With strict adherence to these protocols and guidelines, there was no transmission of COVID-19 to other persons within the OR. We hope that this report will help other hospitals prepare for COVID-19 outbreaks in limited situations.
Notes
Ethical statements
The study was approved by the Institutional Review Board (IRB) of the Kyungpook National University Chilgok Hospital (IRB No. 2020-04-038). The IRB waived patient consent because of the retrospective nature of the study. Patient confidentiality was maintained throughout the study.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Author contributions
Conceptualization: JY, KHK, KTK; Data curation: JKK; Ivestigation: JKK; Visualization: JKK, JY; Writing - original draft: JKK, JY; Writing - review & editing: JKK, KTK, JY.