Pelvic floor muscle exercise with biofeedback helps regain urinary continence after robot-assisted radical prostatectomy
Article information
Abstract
Background
To determine the benefit of pelvic floor muscle exercise (PFME) with visual biofeedback on promoting patient recovery from incontinence, we investigated variables associated with the early restoration of continence for patients who robot-assisted radical prostatectomy (RARP).
Methods
Of the 83 patients enrolled, 41 consecutive patients completed PFME (the exercise group), and the other 42 consecutive patients just before the PFME program commenced (the control group). The primary outcome was whether PFME engagement was associated with zero pad continence restoration within 3 months of surgery.
Results
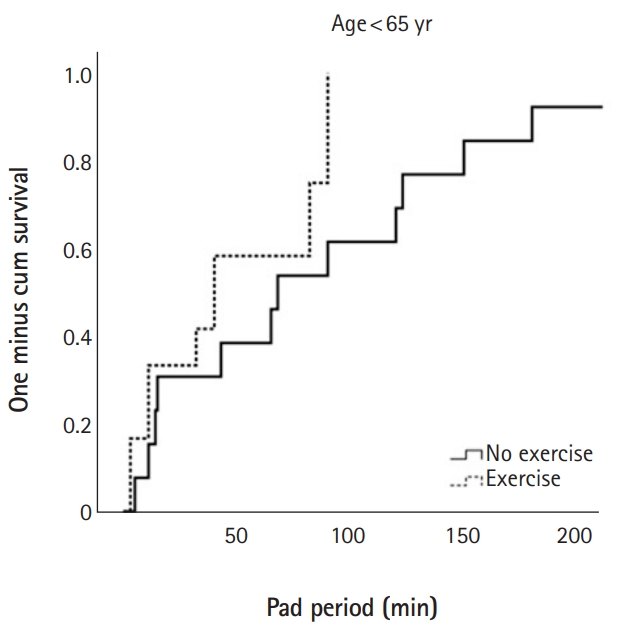
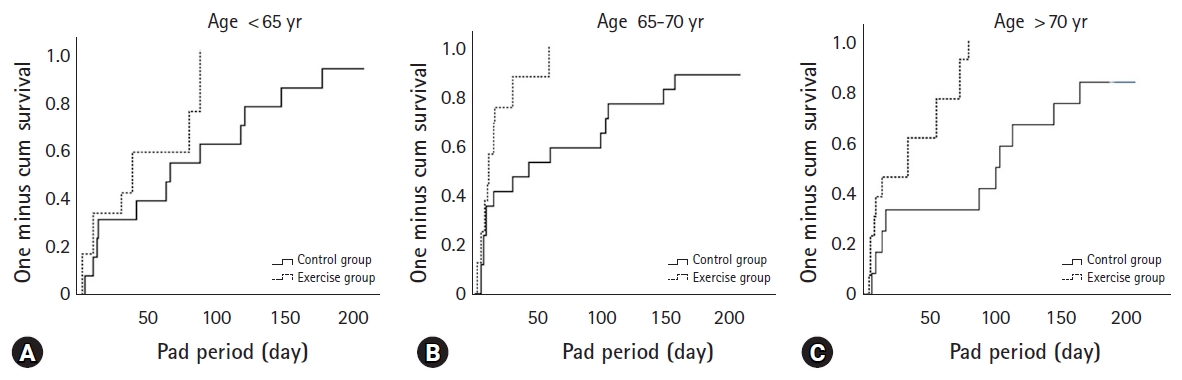
Continence restoration percentages (defined as zero pads used per day) at 1, 3, and 6 months after surgery were 49.4%, 77.1%, and 94.0%, respectively. The exercise group achieved significantly higher recovery rates at 1 month (p=0.037), 3 months (p<0.001), and 6 months (p=0.023). Cox regression analysis demonstrated that a lower Gleason score (<8; hazard ratio [HR], 2.167), lower prostate specific antigen (<20 ng/dL; HR, 2.909), and engagement in PFME (HR, 3.731) were independent predictors of early recovery from postprostatectomy incontinence. Stratification by age showed that those younger than 65 years did not benefit significantly from exercise (log-rank test, p=0.08), but that their elderly counterparts, aged 65–70 years (p=0.007) and >70 years old (p=0.002) benefited significantly.
Conclusion
This study suggests that postoperative engagement in PFME with biofeedback speeds up the recovery of continence in elderly patients (≥65 years old) that undergo RARP.
Introduction
Radical prostatectomy (RP) is a standard treatment for non-metastatic prostate cancer [1]. Increased enthusiasm for radical organ removal as a reliable treatment option for high-risk or locally advanced disease, combined with the minimal invasiveness unique to robot-assisted radical prostatectomy (RARP), has resulted in RP becoming the contemporary treatment of choice. However, postprostatectomy incontinence (PPI) remains a serious issue that diminishes postoperative quality of life [2]. The majority of patients experience urinary incontinence immediately after RP, and in some cases this incontinence is protracted. As such, urinary incontinence is a major source of concern for patients requiring RP or RARP.
As a postoperative intervention, pelvic floor muscle exercise (PFME), with or without biofeedback, is known to promote muscle contraction and hasten recovery from PPI. Although initial trials produced promising results, systematic reviews have led to questions regarding the efficacy of PFME [3]. Furthermore, no large-scale randomized controlled trials (RCTs) have been conducted to test the efficacy of PFME with biofeedback, although a trial is underway [4]. In addition, published evidence regarding the efficacy of existing PFME programs for preventing and treating PPI is inconsistent [5].
In an attempt to identify factors that hasten the restoration of continence, we compared outcomes between patients who engaged in PFME with visual biofeedback and patients who engaged in the Kegel exercise with verbal instructions alone. Given uncertainties regarding the benefits of PFME, we minimized confounding factors by using data from patients treated by a single surgeon, using the same RARP technique over a period of 1 year.
Material and methods
1. Recruitment of the exercise and study groups
This study was approved by the Institutional Review Board of the Yeungnam University Hospital (IRB No: 2020-05-001).
All patients that underwent RARP, performed by a single experienced surgeon (YHK) from September 2018 to August 2019, were enrolled in this study. During RARP three procedures were performed: unilateral, bilateral, or no nerve-sparing; bladder neck preservation; and posterior reconstruction. The same surgical techniques were used throughout the 12-month study period. The study exclusion criteria were as follows: previous pelvic radiation therapy, poor compliance due to psychiatric or medical problems, previous prostate surgery, and <3 months of follow-up after surgery. Of the 94 patients initially considered, two men that required a cardiac procedure after RARP and nine that were followed for <3 months were excluded. Accordingly, 83 participants constituted the study cohort. Forty-one of the 83 participants (49.4%) engaged in PFME with biofeedback (the exercise group), while the other 42 did not (the control group), as they underwent RARP using the same technique just before the PFME program was adopted.
In the exercise group, PFME with biofeedback was performed by a single physiotherapist (DGL) on patients that underwent RARP between March 2019 and August 2019. Patients in the control group underwent RARP between September 2018 and February 2019 and performed the Kegel exercise at home after being given oral instructions by a urologist (YHK).
2. Pelvic floor muscle exercise
Patients in the exercise group began engaging in PFME immediately after Foley catheter removal, which was routinely performed 5 days after RARP. Patients received PFME with biofeedback on an outpatient basis for 30 minutes per week until continence was regained or 4 weeks had elapsed. Ultrasonography was used to visualize pelvic floor muscle contractions. Patients were asked to perform 20–25 contractions with durations from 3–5 seconds at submaximal strength in the lateral decubitus, supine (with hips flexed at 60°), and standing positions. A relaxation period of 6–10 seconds was allowed between contractions. The physiotherapist checked pelvic floor muscles by palpating the perineum, examined contractions via ultrasonography in each position, and showed patients how to contract pelvic floor muscles correctly on avoiding Valsalva maneuver. In addition, patients were asked to repeat the exercise at home. Patients in the control group were given verbal instructions on the Kegel exercise by a single urologist and asked to perform 50–100 exercises daily at home while lying, sitting, and standing.
3. Outcome assessments
Postoperatively, all 83 patients were routinely followed-up in an outpatient office at 1 week and 1, 3, and 6 months after surgery. During each visit, patients were asked about daily pad use and the last date of pad usage. Continence in this study was strictly defined as the cessation of pad use, regardless of the type of pad used. The primary outcome of this study was the determination of whether PFME with biofeedback impacts the restoration of continence within the 3 months following RARP.
4. Statistical analysis
Group clinicopathological characteristics were compared using Student t-test for continuous variables and a chi-squared test for categorical variables. Given the well-documented association between time and recovery from PPI, the Cox proportional hazards model was used to identify predictors of early continence restoration. The Kaplan-Meier method with the log-rank test was used to compare groups with respect to time to continence. Data were analyzed using IBM SPSS version 19.0 (IBM Corp., Armonk, NY, USA). All tests were two-sided and p-values <0.05 were considered statistically significant.
Results
1. Participant characteristics
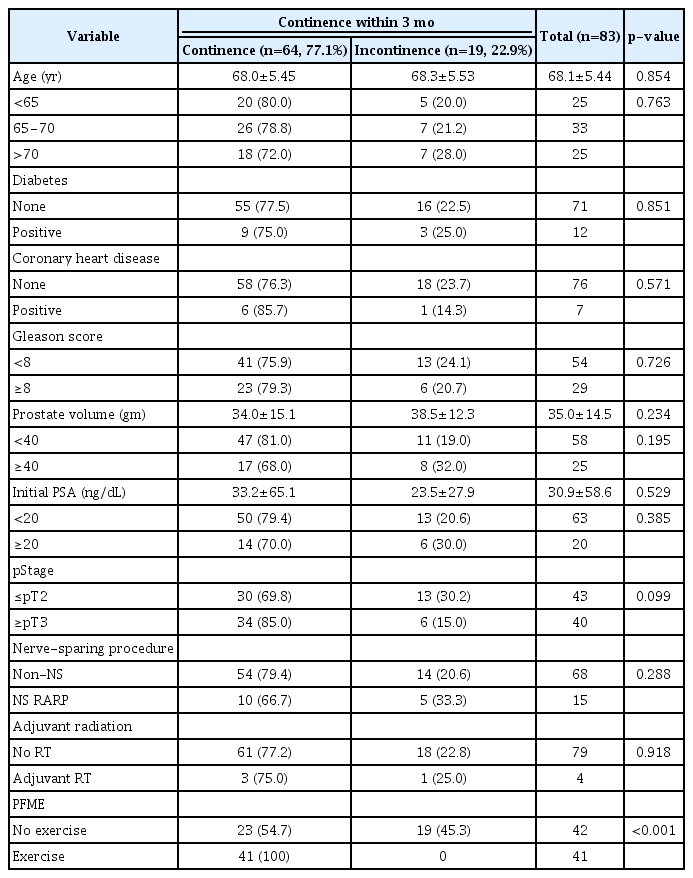
The characteristics of patients in the exercise (PFME with biofeedback) and the control (conventional Kegel exercise) groups are summarized in Table 1. Continuous and categorical variables were similar in the two groups, except initial serum prostate specific antigen (PSA) levels, which were significantly higher in the exercise group (p=0.025). However, the proportions of patients with a PSA value ≥20 ng/dL (the cut-off for high-risk disease) was similar (p=0.276) across groups. The proportion of patients with a Gleason score >8 was marginally higher in the exercise group (p=0.09).
2. Continence outcomes and variables associated with early continence restoration
Continence restoration rates for all study subjects at 1 week, and 1, 3, and 6 months after surgery were 18.1%, 49.4%, 77.1%, and 94.0%, respectively. The exercise group had higher rates of continence restoration than controls at 1 month (p=0.037), 3 months (p<0.001), and 6 months (p=0.023) (Table 2). Three months after surgery, all 41 patients in the exercise group had regained continence. Furthermore, the mean time to restored continence was significantly shorter in the exercise group (32.4 vs. 95.3 days, p<0.001) (Fig. 1). Other than the implementation of PFME instructions, no factors differed between participants who had or had not regained continence after 3 months (Table 3).
3. Multivariate analysis of early continence restoration and post-hoc analysis by age
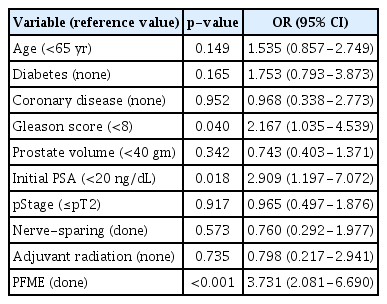
Cox proportional hazards analysis demonstrated that a lower Gleason score (<8; hazard ratio [HR], 2.167), a lower initial PSA (<20 ng/dL; HR, 2.909), and PFME completion (HR, 3.731) were associated with continence restoration within 3 months of RARP (Table 4). After stratifying all study subjects by age, those aged <65 years were found to receive no significant benefit from exercise (log-rank test, p=0.08) (Fig. 2A). In contrast, patients ranging from 65–70 years of age (p=0.007) (Fig. 2B) and those older than 70 years (p=0.002) (Fig. 2C) benefited significantly from PFME.
Discussion
The incidence of PPI has been reported to range from as low as 2% to as high as 87% with significant leakage in 0.3%–12.5% of patients at 1 month after surgery [6]. Furthermore, PPI has been reported to last as long as 1–2 years after surgery [7]. The physiological mechanism underlying PPI is multifactorial and has been attributed, in part, to damage to the sphincter. This structure is composed of smooth inner muscles and the rhabdo-sphincter muscle [8-10]. PPI also compromises a supporting system, including Denonvilliers’ fascia, the puboprostatic ligament, the endopelvic fascia, and the levator ani muscle, during RP [11]. Based on these mechanisms, many intraoperative interventions have been devised to reduce the severity and incidence of PPI. These include bladder neck preservation, posterior reconstruction, and a nerve-sparing procedure [12-14].
PFME with or without biofeedback is a type of postoperative intervention that improves urinary continence after RP. According to the European Association of Urology guidelines, PFME with or without biofeedback is the recommended option for conservative management of PPI [15]. Theoretically, PFME improves sphincter function by enhancing rhabdosphincter tone and strengthening levator ani muscles [16]. Several studies have reported on the short- and long-term effects of PFME with biofeedback. Ribeiro et al. [17] conducted an RCT of 73 patients and demonstrated that early PFME with biofeedback after RARP was superior to conventional PFME, as measured by the continence recovery rate at 1 year after surgery (96% vs. 75%; p=0.028). Burgio et al. [18] suggested that preoperative behavioral training can reduce time to recovery of urine control and reduce the severity of incontinence after RP. On the other hand, Bales et al. [19] reported that continence restoration at 1, 2, 3, and 4 months after surgery was not significantly different between biofeedback and control groups. Overgard et al. [20] carried out an RCT of 85 patients and found that early continence restoration rates were similar in a PFME with biofeedback group and in a control group (46% vs. 43%, p=0.73). Therefore, the efficacy of perioperative PFME with biofeedback remains a controversial topic.
We defined continence as the cessation of daily pad use. Several studies defined continence as the use of a single safety or 0 pads daily [17,20], whereas others defined it based on 1 hour or 24 hours pad test results, as recommended by the International Continence Society [21]. We believe that defining continence based on pad usage is sufficient for the assessment of continence and more convenient for patients than the pad test.
Unfortunately, in some studies of the efficacy of PFME after RP, many factors that might predispose patients to PPI, such as type of surgery or number of surgeons, were not controlled. In a study by Geraerts et al. [22], RP was performed by open surgery or RARP and patient numbers between these two groups were unequal (open=116 vs. RARP=54), and in a multicenter study by Floratos et al. [23], radical prostatectomies were conducted by any of four experienced surgeons. These factors can lead to statistical errors when assessing the efficacy of PFME for PPI. On the other hand, the present study was conducted at a single institute by a surgeon who had experience with more than 200 cases.
Additionally, we investigated the independent factors including PFME with biofeedback for early continence restoration, based on the days of pads used. In the majority of previous studies on the effectiveness of PFME with biofeedback, predictive factors, such as age, receipt of a nerve-sparing procedure, and type of surgery, were not considered. We believe that it will be statistically persuasive to establish the relationship between early continence restoration and other clinic-pathological factors for PPI, including PFME with biofeedback, because these factors could also affect PPI recovery rates.
In our study, PFME with biofeedback had a more positive impact on PPI in elderly men (>70 years old) than in younger men. In a systematic review of factors that contribute to PPI, age was found to have a negative impact on continence rate after RP [24]. Simard and Tu [25] reported that pelvic floor muscle rehabilitation with physiotherapy was effective in elderly women with urinary incontinence. However, no study has addressed the relationship between age and the efficacy of PFME with biofeedback. Based on the results of this study, we suggest that PFME with biofeedback for patients with PPI is more effective in elderly men.
The present study has a number of limitations that deserve consideration. First, the number of patients enrolled was relatively small and the study was inherently limited by its retrospective design. However, by adopting data derived from a single surgeon’s experience, we sought to minimize the influence of potential covariates. Second, we focused on early recovery, and did not investigate the long-term effects of PFME with biofeedback, because experience has shown that the benefits of PFME with biofeedback manifest during the earlier stages of recovery. Third, self-questionnaires, such as the International Prostate Symptom Score or International Consultation on Incontinence Questionnaire, were not used, although the majority of recent studies have used self-questionnaires to determine outcomes. Therefore, we suggest that further larger-scale studies be conducted to assess the efficacy of PFME with biofeedback, especially in elderly men.
In conclusion, PFME with visual biofeedback was found to be an effective intervention for promoting early continence restoration (within 3 months) in patients that suffered from urinary incontinence after RARP. Furthermore, this study showed that PFME with biofeedback more effectively results in early continence restoration, after RARP, in elderly men.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: all authors; Formal analysis: YUK; Methodology: DGL; Project administration, Supervision: YHK; Investigation: YUK; Writing-original draft: YUK; Writing-review & editing: YHK.