PubMed Central, CAS, DOAJ, KCI

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Volume 40(Suppl); 2023 > Article
-
Original article
Surgical results of only antegrade del Nido cardioplegia infusion in conventional coronary artery bypass grafting: a retrospective study -
Sang-Uk Park
 , Yo Han Bae
, Yo Han Bae , Yun Seok Kim
, Yun Seok Kim , Kyungsub Song
, Kyungsub Song , Woo Sung Jang
, Woo Sung Jang
-
Journal of Yeungnam Medical Science 2023;40(Suppl):S23-S28.
DOI: https://doi.org/10.12701/jyms.2023.00283
Published online: June 28, 2023
Department of Thoracic and Cardiovascular Surgery, Keimyung University School of Medicine, Daegu, Korea
- Corresponding author: Woo Sung Jang, MD, PhD Department of Thoracic and Cardiovascular Surgery, Keimyung University Dongsan Medical Center, Keimyung University School of Medicine, 1035 Dalgubeol-daero, Dalseo-gu, Daegu 42601, Korea Tel: +82-10-6531-3217 • Fax: +82-53-258-4783 • E-mail: whiteuri09@gmail.com
Copyright © 2023 Yeungnam University College of Medicine, Yeungnam University Institute of Medical Science
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 826 Views
- 38 Download
Abstract
-
Background
- Additional retrograde cardioplegia infusion in conventional coronary artery bypass grafting (CABG) was introduced to address the concern of inappropriate cardioplegia delivery through the stenotic coronary artery. However, this method is complex and requires repeated infusions. Therefore, we investigated the surgical outcomes of only antegrade cardioplegia infusion in conventional CABG.
-
Methods
- We included 224 patients who underwent isolated CABG between 2017 and 2019. The patients were divided into two groups according to the cardioplegia infusion method: antegrade cardioplegia infusion with del Nido solution (n=111, group I) and antegrade+retrograde cardioplegia infusion with blood cardioplegia solution (n=113, group II).
-
Results
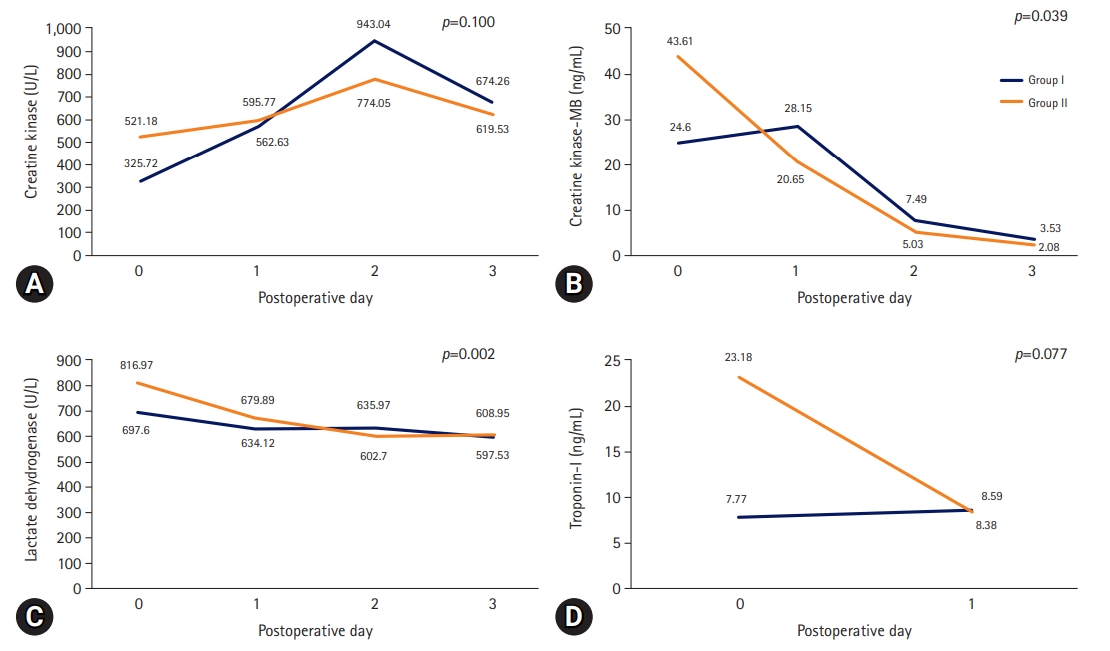
- The sinus recovery time after release of the aorta cross-clamp was shorter in group I (3.8±7.1 minutes, n=98) than in group II (5.8±4.1 minutes, n=73) (p=0.033). The total cardioplegia infusion volume was lower in group I (1,998.6±668.6 mL) than in group II (7,321.0±2,865.3 mL) (p<0.001). Creatine kinase-MB levels were significantly lower in group I than in group II (p=0.039). Newly developed regional wall motion abnormalities on follow-up echocardiography were detected in two patients (1.8%) in group I and five patients (4.4%) in group II (p=0.233). There was no significant difference in ejection fraction improvement between the two groups (3.3%±9.3% in group I and 3.3%±8.7% in group II, p=0.990).
-
Conclusion
- The only antegrade cardioplegia infusion strategy in conventional CABG is safe and has no harmful effects.
- Conventional coronary artery bypass grafting (CABG) is a standard therapy; other types of CABG methods include off-pump and on-pump beating CABG. In conventional CABG, the aorta is cross-clamped and antegrade cardioplegia is infused through each coronary ostium to pause beating and protect the myocardium. In conventional CABG, retrograde cardioplegia is also infused through the coronary sinus to ensure that cardioplegia is properly delivered to the myocardium of patients with coronary artery stenosis. However, this method requires repeated infusions and increases complexity of the surgical field. Thus, we investigated the surgical results of antegrade cardioplegia infusion alone for myocardial protection in conventional CABG.
Introduction
- Ethical statements: The study protocol was approved by Institutional Review Board of Keimyung University Dongsan Medical Center (IRB No: DSMC 2022-08-067-002), and all procedures were performed in accordance with our institutional guidelines for the protection of patient confidentiality. The requirement for patient consent was waived due to the retrospective nature of the study.
- 1. Study population
- Two hundred and twenty-four patients (173 males, 51 females) who underwent isolated CABG between January 2017 and December 2019 were included in this study. The patients were divided into two groups according to the cardioplegia infusion method, which was performed according to the surgeon’s preference: group I, antegrade cardioplegia infusion using del Nido solution (n=111), group II, antegrade+retrograde cardioplegia infusion using blood cardioplegic solution (n=113).
- 2. Data collection
- Data on demographics, clinical and laboratory findings, and clinical outcomes were obtained from the electronic medical records using data collection forms. The demographic data included age, sex, and predefined comorbidities (hypertension, diabetes, chronic obstructive pulmonary disease, and chronic kidney disease). Preoperative and postoperative ejection fraction (EF) was measured by transthoracic echocardiography. Left ventricular dysfunction was defined as EF of <40%. The laboratory data included cardiac markers. The clinical outcomes included echocardiographic data, morbidity, and mortality.
- 3. Surgical technique
- All surgeries were performed after median sternotomy. After graft harvesting, an arterial cannula was inserted into the ascending aorta and a single venous cannula was inserted into the right atrium. Antegrade cardioplegia was infused through the root cannula and retrograde cardioplegia was infused through the coronary sinus after antegrade cardioplegia infusion.
- 4. Cardioplegia strategy
- Del Nido redosing was planned 90 minutes after the initial dose if the total aortic cross-clamp (ACC) time was expected to exceed 120 minutes. Myocardial temperature was not measured, and topical hypothermia was used for all patients.
- Blood cardioplegia was administered in a 4:1 blood dilution as an initial anterograde and/or retrograde bolus of 1,000 to 2,000 mL at 4°C; anterograde or retrograde delivery was repeated every 15 to 20 minutes. In most cases, only a warm shot was delivered retrogradely, according to the surgeon’s preference.
- 5. Patient follow-up
- All patients were evaluated using transthoracic echocardiography before discharge, and EF, EF changes, and newly developed regional wall motion abnormalities were assessed. Cardiac enzyme levels, including creatine kinase (CK), CK-MB, troponin I, and lactate dehydrogenase, were measured for 3 days during the preoperative to postoperative intensive care unit stay. The vasoactive inotropic score (VIS) was calculated as follows: dopamine dose (µg/kg/min)+dobutamine dose (µg/kg/min)+100×epinephrine dose (µg/kg/min)+10×milrinone dose (µg/kg/min)+10,000×vasopressin dose (unit/kg/min)+100×norepinephrine dose (µg/kg/min).
- 6. Statistical analysis
- All continuous variables are expressed as mean±standard deviation, as appropriate. As the number of missing data points was small, missing data were excluded from the analysis. Categorical variables are expressed as frequencies and percentages. Comparisons between continuous variables were performed using Student t-test, and categorical variables were compared using Pearson chi-square tests. We employed a linear mixed model to compare parameters according to the time interval. A p-value of <0.05 was considered to indicate a statistically significant difference. All analyses were performed using IBM SPSS ver. 26.0 (IBM Corp., Armonk, NY, USA).
Methods
- 1. Clinical outcomes
- The mean ages of the two groups were 65.8±9.3 years and 64.4±9.1 years (p=0.254). There were no significant differences in patient characteristics between the two groups (all p>0.05, Table 1). In particular, preoperative EF (p=0.412) and the number of left ventricle dysfunctions (p=0.704) were similar among patients. Patients with a preoperative diagnosis of ST-elevation myocardial infarction, non-ST-elevation myocardial infarction, or unstable angina showed no significant differences (all p>0.05). However, the number of patients diagnosed with stable angina was higher in group I (p=0.011). The mean anastomosis vessel numbers were 2.87±0.85 in group I and 2.72±0.78 in group II (p=0.206), and there was no significant difference in anastomosis target vessels between the two groups (all p>0.05, Table 2).
- 2. Operation records
- Group I showed a shorter mean cardiopulmonary bypass time (101.6±33.1 minutes) than group II (112.5±35.5 minutes) (p=0.019). There was no significant difference in ACC time between the two groups (77.2±25.0 minutes in group I and 76.9±23.4 minutes in group II, p=0.923) or the incidence of defibrillation after ACC due to ventricular fibrillation between the two groups (one in group I [0.9%] and three in group II [2.7%], p=0.322). The sinus recovery time after release of the ACC was shorter in group I (3.8±7.1 minutes, n=98) than in group II (5.8±4.1 minutes, n=73) (p=0.033), and the total cardioplegic infusion volume was lower in group I (1,998.6±1,668.6 mL) than in group II (7,321.0±2,865.3 mL) (p<0.001). The cardioplegia infusion time was also lower in group I (1.4±0.9 times) than in group II (3.9±1.7 times) (p<0.001).
- 3. Postoperative findings
- There was no significant difference in the mortality rate between the two groups (two patients in group I [1.8%] and five patients in group II [4.4%], p=0.259). The VISs of the two groups for postoperative intensive care unit treatment were similar (2.9±4.6 in group I and 3.0±4.2 in group II, p=0.891). There was no significant difference in the incidence of postoperative acute kidney injury or atrial fibrillation between the groups (p=0.173 and p=0.767, respectively) (Table 3).
- Cardiac enzyme levels were checked until postoperative day 3. The trends of both lactate dehydrogenase (p=0.002) and CK-MB (p=0.039) levels were steadier in group I than in group II (Fig. 1).
- 4. Echocardiographic findings
- Newly developed regional wall motion abnormalities on postoperative follow-up echocardiography were detected in two patients in group I (1.8%) and five patients in group II (4.4%) (p=0.233). There was no significant difference in EF improvement between the two groups (3.3%±9.3% in group I and 3.3%±8.7% in group II, p=0.990) (Table 4).
Results
- We found that performing the antegrade cardioplegia infusion strategy with del Nido cardioplegia alone in conventional CABG was safe, with no harmful effects compared to the antegrade and additional retrograde cardioplegia infusion strategies with blood cardioplegia. In addition, a similar incidence of ventricular fibrillation after ACC was observed between the two groups. VIS, postoperative morbidity, and incidence of newly developed regional wall motion abnormalities were similar between the two groups. However, sinus recovery time after release of the ACC was faster, and cardiac enzyme levels at postoperative follow-up were lower in the “only antegrade cardioplegia infusion with del Nido cardioplegia” group than in the “antegrade and additional retrograde cardioplegia infusion with blood cardioplegia” group.
- Antegrade cardioplegia infusion combined with an intermittent retrograde cardioplegia infusion strategy with blood cardioplegia is commonly performed in conventional CABG surgery. A meta-analysis indicated that blood cardioplegia provides superior myocardial protection compared with crystalloid cardioplegia, including lower rates of low cardiac output syndrome and early CK-MB increase [1]. Additionally, one study found that blood cardioplegia and combined antegrade and retrograde cardioplegia was superior to crystalloid cardioplegia. Furthermore, the antegrade cardioplegia-only method showed noninferiority for postoperative morbidity among patients with left ventricular dysfunction [2].
- Del Nido cardioplegia was developed to optimize myocardial protection during congenital cardiac surgery. The immature myocardium in pediatric patients is highly susceptible to ischemia-reperfusion injury and myocardial damage via the accumulation of intracellular calcium [3,4]. Mannitol-, magnesium-, and lidocaine-based del Nido cardioplegia mechanisms protect against myocardial ischemia-reperfusion injury and calcium-induced hypercontraction [3]. Furthermore, lidocaine in del Nido cardioplegia reduces intracellular calcium levels, allowing superior functional recovery with higher peak cardiac output, systolic pressure, and stroke volume [5-8]. Thus, del Nido cardioplegia in pediatric cardiac surgery has been shown to be safe with single-dose administration for >90 minutes [9]. Recently, del Nido cardioplegia was introduced for use in adult cardiac surgery [10,11] and was found to be safe and have clinical outcomes comparable to those of conventional blood or crystalloid cardioplegia in adult cardiac surgery [11-13]. Moreover, the safety of del Nido cardioplegia extends to CABG surgery using antegrade and retrograde delivery infusion methods [14]. However, studies on the superiority of antegrade cardioplegia infusion with del Nido cardioplegia over conventional blood cardioplegia with antegrade and retrograde infusion in CABG are limited [15]. Indeed, there is only one report on the noninferiority of myocardial protection and clinical outcomes with del Nido cardioplegia versus blood cardioplegia [16]. In our study, we showed noninferiority in VIS, postoperative morbidity, and incidence of newly developed regional wall motion abnormalities by echocardiography. We also observed superiority for faster sinus recovery time and a tendency toward lower cardiac enzyme levels. Thus, excellent outcomes were demonstrated in CABG using only the antegrade cardioplegia infusion strategy with del Nido cardioplegia.
- Our study had several limitations. As the data and characteristics of the patients were retrospectively collected from electronic medical records, the nature of the separate groups was not balanced. Another limitation of our study was its small sample size. This study was designed to compare antegrade infusion of del Nido cardioplegia with antegrade+retrograde infusion of blood cardioplegia. Therefore, the type of cardioplegia and method of delivery could have affected the results. Despite these limitations, we believe that the results of our study can provide strong evidence for cardiac surgeons choosing the “only antegrade cardioplegia infusion with the del Nido cardioplegia” method. This relieves concerns regarding the possibility of cardioplegia delivery failure through the stenotic coronary artery. Further studies with prospective designs, controlled variables, and large sample sizes with balanced patient characteristics are warranted to support our findings.
- In conclusion, compared to blood cardioplegia infusion with antegrade and retrograde infusion methods, an antegrade cardioplegia infusion strategy with del Nido cardioplegia in conventional CABG is simple, safe, and leads to no harmful effects.
Discussion
-
Conflicts of interest
No potential conflict of interest relevant to this article are reported.
-
Funding
None.
-
Author contributions
Conceptualization: WSJ; Data curation: YHB; Formal analysis: WSJ, YHB, KS; Methodology: SUP, YHB, KS; Project administration: WSJ; Visualization: SUP, WSJ; Writing-original draft: SUP, WSJ, YHB; Writing-review & editing: all authors.
Notes
Values are presented as number only, mean±standard deviation, or number (%).
Group I, antegrade cardioplegia infusion using del Nido solution; group II, antegrade+retrograde cardioplegia infusion using blood cardioplegic solution.
COPD, chronic obstructive pulmonary disease; EF, ejection fraction; LV, left ventricle; STEMI, ST-elevation myocardial infarction; NSTEMI, non- STEMI.
Values are presented as mean±standard deviation, number (%), or number only.
Group I, antegrade cardioplegia infusion using del Nido solution; group II, antegrade+retrograde cardioplegia infusion using blood cardioplegic solution.
LAD, left anterior descending; OM, obtuse marginal; RCA, right coronary artery; PDA, posterior descending artery; PLB, posterolateral branch.
a)OM1, first obtuse marginal branch; OM2, second obtuse marginal branch.
Values are presented as number (%) or mean±standard deviation.
Group I, antegrade cardioplegia infusion using del Nido solution; group II, antegrade+retrograde cardioplegia infusion using blood cardioplegic solution.
ICU, intensive care unit; VIS, vasoactive inotropic score; AKI, acute kidney injury; Afib, atrial fibrillation.
- 1. Guru V, Omura J, Alghamdi AA, Weisel R, Fremes SE. Is blood superior to crystalloid cardioplegia?: a meta-analysis of randomized clinical trials. Circulation 2006;114(1 Suppl):I331–8.ArticlePubMed
- 2. Flack JE 3rd, Cook JR, May SJ, Lemeshow S, Engelman RM, Rousou JA, et al. Does cardioplegia type affect outcome and survival in patients with advanced left ventricular dysfunction?: results from the CABG Patch Trial. Circulation 2000;102(19 Suppl 3):III84–9.ArticlePubMed
- 3. Matte GS, del Nido PJ. History and use of del Nido cardioplegia solution at Boston Children’s Hospital. J Extra Corpor Technol 2012;44:98–103.ArticlePubMedPMC
- 4. O’Brien JD, Howlett SE, Burton HJ, O’Blenes SB, Litz DS, Friesen CL. Pediatric cardioplegia strategy results in enhanced calcium metabolism and lower serum troponin T. Ann Thorac Surg 2009;87:1517–23.ArticlePubMed
- 5. Govindapillai A, Hua R, Rose R, Friesen CH, O’Blenes SB. Protecting the aged heart during cardiac surgery: use of del Nido cardioplegia provides superior functional recovery in isolated hearts. J Thorac Cardiovasc Surg 2013;146:940–8.ArticlePubMed
- 6. Newton DJ, McLeod GA, Khan F, Belch JJ. Mechanisms influencing the vasoactive effects of lidocaine in human skin. Anaesthesia 2007;62:146–50.ArticlePubMed
- 7. Heydarpour M, Ejiofor J, Gilfeather M, Stone G, Gorham J, Seidman CE, et al. Molecular genetics of lidocaine-containing cardioplegia in the human heart during cardiac surgery. Ann Thorac Surg 2018;106:1379–87.ArticlePubMedPMC
- 8. Perrelli MG, Pagliaro P, Penna C. Ischemia/reperfusion injury and cardioprotective mechanisms: role of mitochondria and reactive oxygen species. World J Cardiol 2011;3:186–200.ArticlePubMedPMC
- 9. Charette K, Gerrah R, Quaegebeur J, Chen J, Riley D, Mongero L, et al. Single dose myocardial protection technique utilizing del Nido cardioplegia solution during congenital heart surgery procedures. Perfusion 2012;27:98–103.ArticlePubMedPDF
- 10. Ad N. del Nido cardioplegia: ready for prime time in adult cardiac surgery? J Thorac Cardiovasc Surg 2015;149:637–8.ArticlePubMed
- 11. Ad N, Holmes SD, Massimiano PS, Rongione AJ, Fornaresio LM, Fitzgerald D. The use of del Nido cardioplegia in adult cardiac surgery: a prospective randomized trial. J Thorac Cardiovasc Surg 2018;155:1011–8.ArticlePubMed
- 12. Ramanathan R, Parrish DW, Armour TK, Brinster DR. Use of del Nido cardioplegia in adult cardiac surgery. Thorac Cardiovasc Surg 2015;63:624–7.ArticlePubMed
- 13. Guajardo Salinas GE, Nutt R, Rodriguez-Araujo G. Del Nido cardioplegia in low risk adults undergoing first time coronary artery bypass surgery. Perfusion 2017;32:68–73.ArticlePubMedPDF
- 14. Yerebakan H, Sorabella RA, Najjar M, Castillero E, Mongero L, Beck J, et al. Del Nido cardioplegia can be safely administered in high-risk coronary artery bypass grafting surgery after acute myocardial infarction: a propensity matched comparison. J Cardiothorac Surg 2014;9:141.ArticlePubMedPMCPDF
- 15. Timek T, Willekes C, Hulme O, Himelhoch B, Nadeau D, Borgman A, et al. Propensity matched analysis of del Nido cardioplegia in adult coronary artery bypass grafting: initial experience with 100 consecutive patients. Ann Thorac Surg 2016;101:2237–41.ArticlePubMed
- 16. Timek TA, Beute T, Robinson JA, Zalizadeh D, Mater R, Parker JL, et al. Del Nido cardioplegia in isolated adult coronary artery bypass surgery. J Thorac Cardiovasc Surg 2020;160:1479–85.ArticlePubMed
References
Figure & Data
References
Citations


 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine PubReader
PubReader ePub Link
ePub Link Cite
Cite