Thallium poisoning: a case report
Article information
Abstract
Thallium poisoning is usually accidental. We present a case of a 51-year-old woman who was evaluated in June 2018 for myalgia, vertigo, asthenia, and abdominal pain. Physical examination revealed temporal-spatial disorientation, jaundice, and asterixis. The laboratory reported the following: bilirubin, 10.3 mg/dL; aspartate transaminase, 78 U/L; alanine transaminase, 194 U/L; albumin, 2.3 g/dL; prothrombin time, 40%; and platelet count, 60,000/mm3. Serology performed for hepatitis A, B, and C; Epstein-Barr virus; cytomegalovirus; and human immunodeficiency virus was negative, and a collagenogram was negative. Physical reevaluation revealed alopecia on the scalp, armpits, and eyebrows; macules on the face; plantar hyperkeratosis; and ulcers on the lower limbs. Tests for lead, arsenic, copper, and mercury were carried out, which were normal; however, elevated urinary thallium (540 µg/g; range, 0.4–10 µg/g) was observed. The patient was treated with D-penicillamine 1,000 mg/day and recovered her urinary thallium levels were within normal range at annual follow-up. Thallium poisoning is extremely rare and can be fatal in small doses. An adequate clinical approach can facilitate early diagnosis.
Introduction
Thallium is a colorless, odorless, heavy metal present in the air, water, and soil. Its initial use in pesticides led to multiple reports of intoxication and death from exposure, which is why its domestic use has been prohibited since the 1960s, relegating it to limited industrial applications.
Thallium enters the body via digestive, respiratory, and skin routes. It widely distributes, fixing itself in the nervous system, skin and appendages, muscle, liver, kidneys, and adipose tissue, and can cross the placental barrier and cause fetal damage [1].
It does not undergo metabolism and rapidly reaches the enterohepatic circulation, which can prolong its half-life for up to 4 days (occasionally more than 30 days). It is excreted mainly through the kidneys and the digestive tract over several weeks, which explains the presence of high levels of the compound in 24-hour urine tests (the most reliable method) in contrast to the low plasma levels after more than 4 days have elapsed postexposure. The lethal dose of thallium is 10 to 15 mg/kg [2,3].
Although the exact mechanism of its toxicity is still not clear, thallium is known to exchange with intracellular potassium and have a high affinity for the sulfhydryl groups of mitochondrial membrane proteins, resulting in the inhibition of glycolysis and energy production in the Krebs cycle and oxidative phosphorylation [4].
After ingestion of thallium, vomiting and abdominal pain are noted within minutes; dry mouth and diarrhea (or constipation) may persist for days or weeks. Alopecia and encephalopathy (dementia, confusion, ataxia, and coma) are dependent on the amount, duration, and dose of thallium [3,4].
Cases of poisoning are extremely rare and mostly accidental. In the Argentine Republic, 335 thallium poisoning cases were reported from 1977 to 1985, after which the use of this compound was completely prohibited in the manufacture of rodenticides. Since then, there have been no other official reports because the detection of new cases has been extremely rare [5].
Case
Ethical statements: This study was exempt from review by the Institutional Review Board (IRB) of Sanatorio de la Providencia (IRB No: 014-2022). Written informed consent was obtained from the patient to participate in the study.
A 51-year-old female patient, a lawyer by profession, living in a residential area of Buenos Aires, presented with a relative to the emergency department in June 2018 due to myalgia and vertigo that had persisted for 1 month and was associated with abdominal pain, daily emesis (up to three episodes), and progressive asthenia over the previous week. She had a history of irritable bowel syndrome and hypothyroidism (treated with levothyroxine 50 µg daily for 2 years and strictly controlled by an endocrinologist).
Physical examination revealed vital signs within normal parameters, with evidence of temporal-spatial disorientation, incoherent speech, generalized jaundice, and flapping. Her laboratory test results were as follows: total bilirubin, 10.3 mg/dL; direct bilirubin, 5 mg/dL; aspartate transaminase, 78 U/L; alanine transaminase, 194 U/L; prothrombin time, 40%; international normalized ratio, 1.7; albumin, 2.3 g/dL; platelet count, 60,000/mm3; lactate dehydrogenase, 1,130 U/L; and C-reactive protein, 69 mg/dL. The remaining initial tests, including sodium, potassium, calcium, phosphorus, magnesium, ammonium, and chlorine levels, were unremarkable. Likewise, brain computed tomography was performed without contrast, which did not reveal morphological compromise of the central nervous system.
The patient was hospitalized on the basis of a diagnosis of acute liver failure. Serology was performed for hepatitis A, B, and C; Epstein-Barr virus; cytomegalovirus; human immunodeficiency virus; and antibodies to detect autoimmune disorders (antimitochondrial antibody, anti-liver-kidney microsomal antibody, smooth muscle antibody, and factor associated with neutral sphingomyelinase activation), which were all negative. It was decided to perform a thyroid profile based on the patient’s pathological history, revealing normal values (thyroid-stimulating hormone, 1.9 µU/mL; free thyroxine, 70 nmol/L; and triiodothyronine, 2 nmol/L).
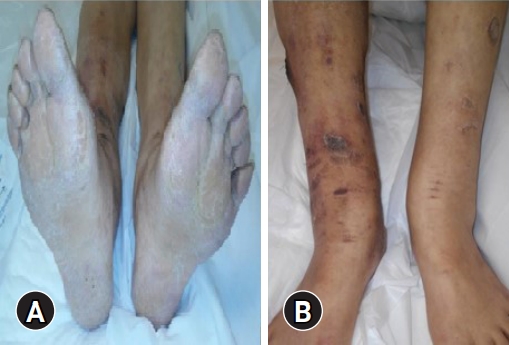
After a second physical evaluation, the following were found: alopecia on the scalp, armpits, and eyebrows (Fig. 1); brown macules on the face; dry and rough skin with anhidrosis; generalized hypotonia; plantar hyperkeratosis (Fig. 2A); and ulcers on both limbs (Fig. 2B), in addition to weakness and hyporeflexia associated with decreased sensitivity in the four extremities. When the patient was again questioned to obtain another history of exposure, she denied the use of alcoholic beverages, tobacco, therapy with herbs or plants, direct contact with insecticides or poisons, or consumption of medications other than levothyroxine. She stated that she had always lived within the same area, which was strictly residential without nearby factories or industrial activity. She also said that she was unaware of any reports of poisoning in people in her close circle or the appearance of symptoms similar to hers in the people with whom she lived or in the neighbors with whom he had constant communication.
Brain computed tomography and abdominal ultrasound were repeated, which did not show any pathological findings. Due to suspicion of intoxication by heavy metals, they were measured in her plasma (p) and 24-hour urine (u), resulting in the following values: p lead, 5.5 µg/dL (range, <30 µg/dL); p arsenic, 0.4 µg/dL (range, ≤0.4 µg/dL); u arsenic, <0.1 µg/g creatinine (Cr) (range, ≤10 µg/g Cr); p copper, 68 µg/dL (range, 70–160 µg/dL); u copper, 57.4 µg/24 hr (range, 15–64 µg/24 hr); p mercury, <0.5 µg/L (range, <15 µg/L); u mercury, 2.6 µg/g Cr (range, <50 µg/g Cr); p thallium, 12 µg/dL (range, ≤80 µg/dL); and TLU, 540 µg/g Cr (range, 0.4–10 µg/g Cr).
After making the diagnosis of chronic thallium intoxication, chelation therapy was started with oral D-penicillamine 250 mg every 6 hours. The patient presented a favorable evolution after 2 weeks of treatment and was discharged. Monthly follow-ups were carried out, in which an average decrease in TLU levels of 10% was observed at each appointment; all symptoms (including alopecia) resolved after 6 months of treatment.
After 1 year of treatment, her TLU levels were measured and found to be within normal limits (2.3 µg/g Cr). At the date of this publication, the source of poisoning is still unknown.
Discussion
The findings presented in our clinical case are consistent with those of other cases published in the literature, in which the appearance of a characteristic triad has been shown, leading to the suspicion of thallium intoxication [2,6,7]. This triad consists of the presence of abdominal pain, evidence of a motor or sensory neurological deficit, and alopecia. The pattern of presentation usually develops in the following order after exposure to thallium: nausea, vomiting, and abdominal pain appearing during the first hours after exposure, followed by the appearance of peripheral sensorimotor neuropathy after 72 or more hours, and finally generalized alopecia weeks later (Table 1). Thus, based on the data mentioned in Table 1, as well as the clinical manifestation of our patient, it can be inferred that these symptoms are related to thallium intoxication that has progressed between 2 and 4 weeks [8-10].
Although thallium affects organs in a generalized way, the liver is one of the most affected. Acute liver failure has not been reported as an isolated symptom, but rather as part of multi-organ toxicity. As part of the neurological involvement, painful peripheral neuropathy (“burning feet syndrome”) was the most frequently described, which differs from this case in which the neuropathy was characterized as hypoesthesia and was not painful [11,12].
With regard to treatment, it is now known that the antidote for thallium intoxication is Prussian blue (3 g daily for adults), usually administered with a cathartic for better adsorption and elimination of the metal. Despite this, the available data are insufficient to comment definitively on its effectiveness. Thus, the management of thallium intoxication does not seem to be systematized because it is subject to multiple controversies [13]. In this case, D-penicillamine was chosen as the chelating treatment for several reasons. First, D-penicillamine was immediately available, as opposed to Prussian blue. Second, the patient’s chronic constipation contraindicated the use of Prussian blue. Third, Prussian blue is recommended for acute thallium poisoning.
We considered the importance of presenting this case because thallium poisoning can be fatal in small doses in a very short period of time. In addition, such cases are extremely infrequent and have a highly variable clinical presentation, resulting in many misdiagnoses. Because of this, it is necessary to understand the clinical manifestations of thallium poisoning, with a high index of suspicion involving the presence of alopecia (a characteristic sign) associated with various manifestations, the most frequent being gastrointestinal symptoms in the early stage and nervous system alterations in the late stage.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Author contributions
Conceptualization: all authors; Investigation: OJ, LS; Formal analysis, Supervision: HC, LG, JAAR; Resources: OJ, HC, MM; Validation, Visualization: MM; Writing-original draft: JAAR; Writing-review & editing: OJ, LG, LS, JAAR.