Hypertension and cognitive dysfunction: a narrative review
Article information
Abstract
Cognitive dysfunction is relatively less considered a complication of hypertension. However, there is sufficient evidence to show that high blood pressure in middle age increases the risk of cognitive decline and dementia in old age. The greatest impact on cognitive function in those with hypertension is on executive or frontal lobe function, similar to the area most damaged in vascular dementia. Possible cognitive disorders associated with hypertension are vascular dementia, Alzheimer disease, and Lewy body dementia, listed in decreasing strength of association. The pathophysiology of cognitive dysfunction in individuals with hypertension includes brain atrophy, microinfarcts, microbleeds, neuronal loss, white matter lesions, network disruption, neurovascular unit damage, reduced cerebral blood flow, blood-brain barrier damage, enlarged perivascular damage, and proteinopathy. Antihypertensive drugs may reduce the risk of cognitive decline and dementia. Given the high prevalence of dementia and its impact on quality of life, treatment of hypertension to reduce cognitive decline may be a clinically relevant intervention.
Introduction
Hypertension is a serious medical condition that significantly increases the risk of cardiovascular, cerebral, renal, and other organ dysfunction [1]. Cognitive impairment is comparatively less considered to be an adverse effect of hypertension. However, accumulating evidence supports the causal role of hypertension in cognitive decline beyond its relationship with stroke [2]. Cognitive decline in old age is regarded as an irreversible condition due to degenerative changes, and in many cases, it reduces quality of life and is difficult to treat in diagnosed patients [3]. However, this cognitive decline is affected by many other factors in addition to normal age-related degenerative changes, and hypertension is one of the most important risk factors because it can be controlled and modified and has a high prevalence [4]. Therefore, useful research evidence should be obtained to review and summarize the cognitive dysfunction in patients with hypertension. The purpose of this study is to examine the characteristics of cognitive dysfunction in patients with hypertension and to summarize studies showing the association of hypertension with cognitive disorders and how hypertension causes pathophysiological changes in the brain that lead to cognitive dysfunction.
Epidemiological evidence
Epidemiological data from the Framingham study [5] suggest that there is no association between blood pressure (BP) and co-measured cognitive ability. However, a longitudinal reanalysis of the data showed that the 20-year mean BP was inversely related to cognitive ability [5]. A midlife hypertension and 20-year cognitive cohort study demonstrated that only high systolic BP in midlife, not increased systolic BP in late life was associated with more cognitive decline during the 20-year study [6]. Other cohort studies have also shown that midlife hypertension is a significant predictor of both cognitive dysfunction and morphological changes in the brain [7,8]. A retrospective cohort study including 721 individuals also found that midlife hypertension was associated with late-life dementia [9,10]. The Cardiovascular Risk Factors, Aging, and Dementia (CAIDE) study, including 1,449 participants aged 65 to 79 years with an average follow-up of over 21 years, suggested that midlife hypertension was associated with an increased risk of dementia in late life [11]. Another study indicated that hypertension in middle age increases the risk of mild cognitive impairment (MCI). A population-based study, using the same cohort of CAIDE study, suggested that midlife hypertension is associated with the development of MCI in late life [12]. The Atherosclerosis Risk in Communities (ARIC) cohort study showed that hypertension and prehypertension in midlife are associated with an increased risk of dementia in late life [9]. The association between midlife hypertension and dementia was also suggested by the Honolulu-Asia Aging Study of 3,703 participants, which showed a consistent association between Alzheimer disease (AD) and vascular dementia [13]. A recent meta-analysis that included 209 prospective studies also showed that midlife hypertension has a stronger association than late-life hypertension. In that meta-analysis, midlife hypertension was associated with an excess risk of 1.19 to 1.55 times that of cognitive impairment. The dose-response relationship was also analyzed, and a midlife systolic BP greater than 130 mmHg was associated with an increased risk of cognitive disorders [14]. The follow-up Honolulu-Asia Aging Study, including 2,505 male participants aged 71 to 93 years, showed that pulsatile pressure was not associated with the incidence of dementia; however, midlife systolic BP was the strongest predictor of dementia incidence [15]. Another study investigated the interactive effects of apolipoprotein E ε4 (APOE-ε4) and hypertension on cognitive decline and brain atrophy. In that study, hypertension was associated with early cognitive decline and brain atrophy in mid-to-late life, particularly in APOE-ε4 carriers [16].
In addition, an U-shaped relationship between hypertension and dementia incidence was reported in a longitudinal population-based study [17]. Low diastolic BP in late life is also associated with an increased risk of AD [18].
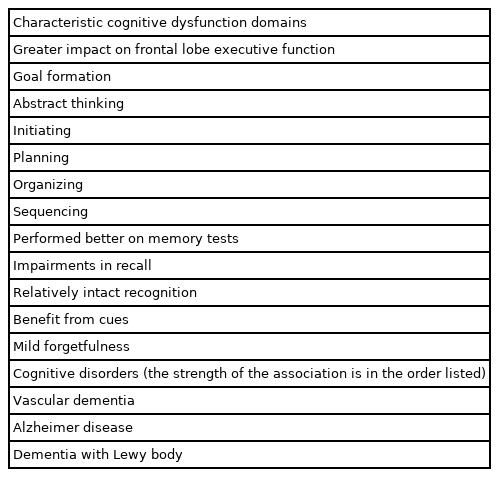
Characteristic cognitive dysfunction domains in hypertension
Cognitive impairments in hypertension can occur across multiple neuropsychological domains, including learning and memory, attention, abstract reasoning, mental flexibility, psychomotor skills, and visuospatial functioning [19]. Although cognitive impairment associated with hypertension may be global, when a more detailed neuropsychological battery is implemented and hypertension-specific effects are considered and compared, the greatest impact of hypertension is on executive function [6], motor speed, and attention [20]. Other studies have reported that hypertensive cerebrovascular disease generally causes damage to the prefrontal subcortex, making it difficult to form goals, abstract, initiate, plan, organize, and sequence [19,21,22]. These characteristic cognitive domains are thought to be involved in subcortical diseases such as classical vascular disease and pure vascular dementia [23]. Although these impairments in executive or prefrontal lobe function usually mask the memory impairment characteristic of AD, impairments in these two cognitive functions often co-occur. Memory impairments in hypertension often tend to be characterized by impairments in recall, but relatively intact recognition, benefit from cues and mild forgetfulness [13].
In a cross-sectional cohort study of 67 patients aged 60 years and older who were hypertensive with MCI or subjective cognitive problems, markers of beta-amyloid retention were associated with worsening episodic memory. In contrast, high white matter intensity, a marker of subcortical ischemic injury, was not associated with performance in any cognitive domain [24]. Because the function of the prefrontal cortex is dependent on the integrity of the cortical-striatal loop through the prefrontal white matter, this type of lesion is very likely to cause a decline in working memory, executive function, and other cognitive abilities assisted by the prefrontal cortex [25]. Therefore, it can be hypothesized that subcortical cerebrovascular disease is more likely to cause cognitive symptoms that are distinguishable from those of AD [26]. Neuropsychological studies of clinically diagnosed patients have reported that compared to patients with AD, patients with vascular dementia performed better on memory tests and poorer on executive function tests. This observation suggests that predominant executive dysfunction may serve as a useful diagnostic marker of vascular dementia. However, a study of 62 autopsy cases showed that major memory impairment was present in 71% of AD cases and predominant executive dysfunction accounted for only 45% of cerebrovascular diseases [26]. In contrast, within large groups that are likely to overlap with the pathology of AD, distinguishing subtypes of dementia based on patterns in impaired cognitive domains is difficult in individuals with mixed MCI and dementia conditions. Finally, a recent meta-analysis showed an association between midlife hypertension and overall cognitive and executive function but not memory [14].
Possible cognitive disorder associated with hypertension
Hypertension has been implicated in various neurocognitive disorders ranging from mild to major [27]. A clear pathophysiological process of vascular dementia in hypertension has been reported [28]. However, hypertension has also been considered a risk factor for AD, although this relationship has not been as clearly elucidated as that between hypertension and vascular dementia [29,30]. In one study, 1,385 participants with a diagnosis of MCI were analyzed to determine whether the degree of high BP was associated with a faster decline in certain cognitive function domains. There were significant main effects of high BP (systolic BP of ≥140 mmHg or diastolic BP of ≥90 mmHg) on neuropsychological measures of visuomotor sequencing, set shifting, and naming. It showed that high BP is associated with a faster decline in cognitive function in people at risk of dementia [31]. Another prospective community-based cohort study also showed that hypertension was related to an increased risk of MCI, and this relationship was stronger in patients with non-amnestic MCI [32].
A significant association was found between frontotemporal dementia and type 2 diabetes [33]. However, in a cohort study, hypertension and other cerebrovascular risk factors were not identified as risk factors for frontotemporal dementia [34]. In a population-based study, hypertension significantly increased the risk of both AD and diffuse dementia with Lewy body (DLB). However, the relationship between hypertension and DLB has been less studied [35]. Finally, a retrospective study investigating BP difference among individuals with different dementia disorders, found that for those patients over 80 years of age, BP did not differ as a function of various dementia disorders [27,34].
In summary, there is strong evidence of an association between vascular dementia and hypertension. However, high BP may contribute to other neurocognitive disorders, such as AD and DLB. Hypertension can negatively affect MCI and shorten the transition to major neurocognitive mpairment (Table 1).
Pathophysiology
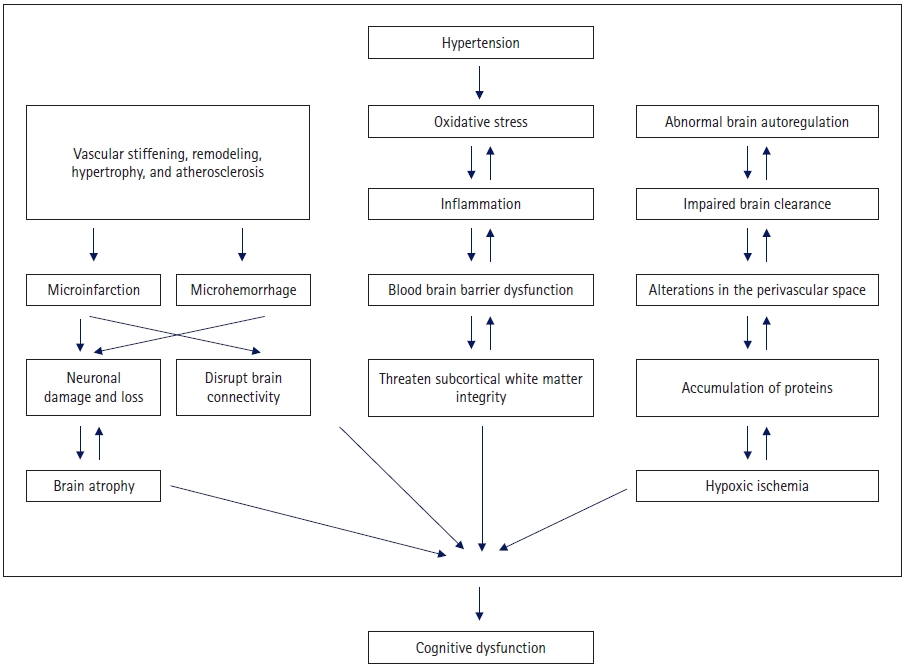
Chronic hypertension is associated with adaptive and degenerative structural changes in the cerebral vessels. These structural changes initially reflect an adaptive response to protect the downstream microvessels from increased transwall pressure. However, this process becomes maladaptive over time. The mechanism of pathological changes associated with hypertension includes vascular remodeling and stiffening, cerebral autoregulation impairment, microbleeding and microinfarction, white matter lesions, lacunar infarction, amyloid angiopathy, and cerebral atrophy [23,36-43]. Brain atrophy, microinfarction, and microhemorrhage can cause nerve cell loss and impaired brain function. In addition, microinfarction and microhemorrhage disrupt brain connectivity and reduce efficiency of the network. Damage to white matter specifically reduces network connectivity, particularly in the thalamic cortical circuit [23,44]. Alterations in the perivascular space can impair brain clearance and promote the accumulation of proteins in the brain and blood vessels. Dysfunction of the neurovascular unit is linked to vascular dysfunction and blood-brain barrier damage [23,45]. Oxidative stress, hypoxic ischemia, inflammation, and blood-brain barrier dysfunction are important vascular factors that threaten the integrity and function of the subcortical white matter. There are possible regional differences in the effects of hypertension on white matter, and white matter in the frontal lobe may be more affected [46,47].
Epidemiological studies have shown that hypertension is a risk factor for vascular cognitive impairment and AD. The effects of hypertension on vascular pathologies have been quite clearly established; however, its impact on AD pathologies is unclear and controversial. In a prospective cohort study of 346 subjects, a cumulative number of midlife vascular risk factors were associated with elevated brain amyloid deposition in late life [48]. However, hypertension itself was not significantly associated with elevated amyloid deposition. Another study evaluating the effect of vascular health on AD imaging biomarkers showed that vascular health had a significantly larger effect on neurodegeneration than on amyloid deposition [49]. In a study of 1,300 deceased participants, some associations were found between systolic BP and neurofibrillary tangles [49,50]. A cerebrospinal fluid biomarker study also found that hypertension was not associated with amyloid-beta 1–42, and that APOE did not have a modifying effect. However, hypertension was directly related to tau, and ptau-181 was modified by the APOE genotype [51]. These findings suggest that hypertension is associated with AD but affects the disease through a pathway different from that involved in amyloid pathology. Unlike clinical studies, experimental studies have suggested that hypertension can contribute to both amyloid and tau pathology in AD, although whether hypertension is a contributor or pathological factor needs to be determined [52-55].
In a longitudinal, prospective, population-based study, men with hypertension who were middle-aged and did not receive antihypertensive treatment had a higher risk of hippocampal atrophy than age-matched men with normal BP. Treatment with antihypertensives reduced the risk associated with these high BPs [56]. These findings suggest that the management of hypertension to lower the risk of AD is important, although the magnitude of the effect of hypertension on AD could be smaller than that on vascular dementia (Fig. 1).
Antihypertensive effects on cognitive function
Data from randomized controlled clinical trials on the efficacy of antihypertensive therapy for the prevention of dementia are contradictory [57-63]. A meta-analysis, including nine randomized controlled trials with 34,994 participants (>60 years) treated for at least 12 months, showed that antihypertensive treatment could reduce cognitive decline with modest effect sizes and did not worsen cognitive dysfunction [64]. Another meta-analysis demonstrated the benefits of antihypertensives. Compared to controls, a drop in BP was significantly associated with a reduced risk of dementia or cognitive impairment (12 trials, 92,135 participants) [65]. A recent review argued that the correlation between BP and cognitive decline was U-shaped and varied according to age. That is, high BP in middle age may be related to cognitive decline in old age, while hypertension in old age may be less related to cognitive decline. In addition, excessively low BP may be associated with cognitive decline; therefore, these U-shaped associations may neutralize the outcomes [18,66]. Subdivided age-specific studies and studies that removed these confounding factors more consistently showed the effects of hypertension on cognitive dysfunction and the ability of antihypertensive drugs to lessen cognitive decline. There is evidence that all effective BP-lowering drugs, including angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, and aldosterone antagonists, can be used to prevent cognitive decline in patients with hypertension and there is no evidence for a difference in effectiveness between these antihypertensives [58,60,67-74]. In addition, a meta-analysis of AD risk factors investigated the relationship between antihypertensive drugs and the risk of cognitive decline. This meta-analysis found that antihypertensive treatment was one of the protective factors for AD with grade I evidence (pooled population of >5,000 individuals) [75].
Conclusion
In this review, we discussed previous studies on hypertension-related cognitive dysfunction. Epidemiological data have shown that midlife hypertension is associated with late-life cognitive impairment. These cognitive disorders encompass both mild and major neurocognitive disorders. Hypertension showed the strongest association with vascular dementia, followed by AD and DLB. Frontotemporal dementia showed no evidence of an association. Hypertension was found to be the most consistent risk factor for dementia only at a certain age; that is, middle-aged hypertension was a risk factor for late-life dementia. In addition, hypotension in old age can be a risk factor for cognitive decline along with hypertension; therefore, BP and cognitive decline have an U-shape relationship. Considering these two characteristics will enable a more consistent interpretation of the mixed research results. Cognitive decline related to hypertension is possible in all areas; however, the decline in executive function related to the frontal lobe is particularly noticeable. This is consistent with the characteristics of vascular dementia, but there are many cases in which AD and vascular dementia coexist in clinical practice, making it difficult to diagnose patients with only these cognitive function characteristics. Many studies have been conducted on the mechanisms by which hypertension causes cognitive decline, including brain atrophy, microinfarcts, microbleeds, neuronal loss, white matter lesions, network disruption, neurovascular unit damage, reduced cerebral blood flow, blood-brain barrier damage, enlarged perivascular damage, and proteinopathy. Medications, lifestyle, and comorbidities, such as diabetes and hyperlipidemia, may have indirect effects in addition to the direct pathophysiology of high BP causing cognitive dysfunction. Hypertension is also a risk factor for other diseases that cause cognitive decline, such as chronic kidney disease or heart failure. Although these factors were identified as confounding factors in the studies included in this review and corrections were made, a more detailed study and review are required in the future. Recent meta-analyses have demonstrated that antihypertensive drugs can reduce the risk of cognitive decline and dementia. Therefore, hypertension is an important risk factor for cognitive decline and dementia and is clinically meaningful because it can be controlled and treated. Considering the high prevalence of both conditions and the serious impact of cognitive function on quality of life, active and timely management of hypertension is required. Additional research is needed, such as a study to determine the optimal BP according to age, which would be helpful for cognitive function, and a therapeutic alternative to address the pathophysiological changes in the brain caused by hypertension.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.