Cerebral fat embolism syndrome: diagnostic challenges and catastrophic outcomes: a case series
Article information
Abstract
Fat embolism syndrome is a rare but alarming, life-threatening clinical condition attributed to fat emboli entering the circulation. It usually occurs as a complication of long-bone fractures and joint reconstruction surgery. Neurological manifestations usually occur 12 to 72 hours after the initial insult. These neurological complications include cerebral infarction, spinal cord ischemia, hemorrhagic stroke, seizures, and coma. Other features include an acute confusional state, autonomic dysfunction, and retinal ischemia. In this case series, we describe three patients with fat embolism syndrome who presented with atypical symptoms and signs and with unusual neuroimaging findings. Cerebral fat embolism may occur without any respiratory or dermatological signs. In these cases, diagnosis was established after excluding other differential diagnoses. Neuroimaging using brain magnetic resonance imaging is of paramount importance in establishing a diagnosis. Aggressive hemodynamic and respiratory support from the beginning and consideration of orthopedic surgical intervention within the first 24 hours after trauma are critical to decreased morbidity and mortality.
Introduction
Fat embolism syndrome is a rare but alarming, life-threatening clinical condition attributed to fat emboli entering the circulation [1]. The first animal model of this syndrome was described over 350 years ago by Lower, who intravenously injected milk into dogs. Magendie performed more elaborate studies in the early 19th century and observed that intravenous injection of oil led to pulmonary edema and the mechanical obstruction of small vessels by fat globules. The first human cases of fat embolism syndrome were described by Zenker in 1862 in patients with severe traumatic crush injuries. He reported the presence of fat droplets in the lung capillaries of railroad workers who died following crush injuries [2]. Since then, fat embolism syndrome has become an interesting research topic, and theories of its pathophysiology and diagnostic criteria have been described [3]. In this case series, we describe three patients with fat embolism syndrome who presented with atypical symptoms, signs, and neuroimaging findings. We also review other atypical neurological presentations of this disorder.
Cases
Ethical statements: This study was approved by the Institutional Review Board (IRB) of King Abdullah International Medical Research Center (IRB No: NRJ22J/143/05). Informed consent was obtained according to the Declaration of Helsinki.
1. Case 1
A 23-year-old male presented to the emergency department with polytrauma sustained during a road traffic injury. He had a thoracic vertebral fracture (T6), left humeral shaft fracture, left femoral shaft fracture, right leg crush injury, and right comminuted femoral fracture. At presentation, his vital signs were normal, and he was awake, alert, and communicating. Two days after admission, he developed confusion, drowsiness, and altered sensorium with a drop in his Glasgow Coma Scale (GCS) score to 9/15. Although a detailed neurological examination was challenging due to confusion and agitation, there were no apparent focal or lateralizing signs. The patient was sedated, intubated, mechanically ventilated, and transferred to the intensive care unit. An urgent brain computed tomography (CT) scan revealed generalized edema with no signs of infarction or hemorrhage. He was administered quetiapine (150 mg twice daily) and clonazepam (0.5 mg once daily). On the sixth day of admission, urgent brain magnetic resonance imaging (MRI) revealed extensive bilateral symmetrical punctate foci of nearly the same size with abnormal signal intensities. These foci affected both the cerebral and cerebellar hemispheres with restricted diffusion, decreased signals in susceptibility-weighted imaging (SWI) indicative of microbleeds, and mottled fluid-attenuated inversion recovery (FLAIR) signals (Fig. 1). The MRI appearance suggested a diagnosis of cerebral fat embolism. The patient endured a stormy course in the intensive care unit for two additional months before being transferred to the orthopedic ward, where he underwent surgery for his fractures. The patient was discharged in a stable condition with follow-up at a multidisciplinary clinic and rehabilitation program.
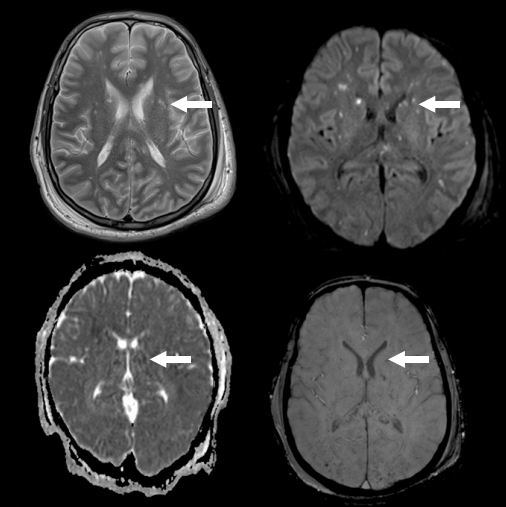
Brain magnetic resonance images show extensive bilateral, symmetrical punctate foci of nearly the same size and abnormal signal intensities affecting both cerebral and cerebellar hemispheres with diffusion restriction and decreased susceptibility-weighted imaging (microbleeds) and mottled fluid-attenuated inversion recovery signals. Arrows indicate one of the foci as an example.
2. Case 2
A 30-year-old male presented to the emergency department 3 hours after a road traffic injury with a right comminuted femoral fracture, left proximal tibial fracture, and left fibular fracture. A few hours later, his level of consciousness started to decline, and he became unresponsive, with a GCS score of 8/15. He also developed oxygen desaturation (O2 of 92%) and was sedated and intubated with subsequent transfer to the intensive care unit. A plain CT scan of the brain was unremarkable, with no ischemia or hemorrhage. Three days later, the patient showed no improvement in the level of consciousness when sedation was stopped. Due to this unexplained drop in his level of consciousness, brain MRI was ordered on the fifth day of admission and revealed extensive small high-signal-intensity lesions of the whole brain, resembling a starfield pattern in T2-weighted and FLAIR images with restricted diffusion and microhemorrhages in SWI (Fig. 2). The patient endured a stormy course in which he remained in the intensive care unit for 1 month with no significant neurological improvement. He underwent orthopedic fixation surgery and was transferred with a tracheostomy to a rehabilitation floor. He continued to improve slowly with the help of a multidisciplinary team and rehabilitation.
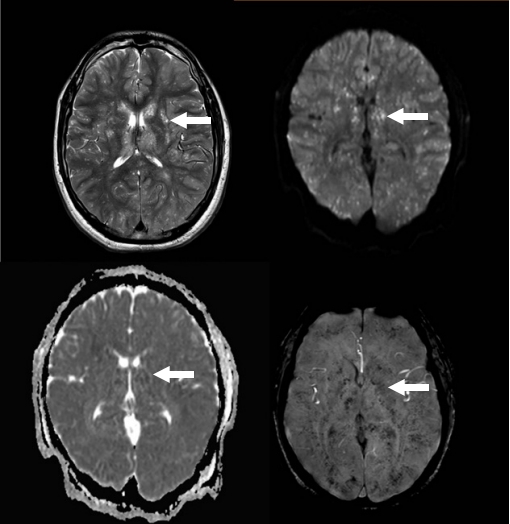
Brain magnetic resonance images show extensive small high-signal intensity lesions involving the whole brain giving the appearance of a starfield pattern on T2-weighted images with restricted diffusion and profuse microhemorrhages in susceptibility-weighted imaging. Arrows indicate one of the lesions as an example.
3. Case 3
A 19-year-old male was admitted after a road traffic injury with multiple fractures (right proximal tibial and right femoral fractures). The patient was conscious, alert, and communicating, with a normal GCS score. A few hours later, he developed acute onset loss of consciousness (GCS score of 3/15) with subsequent sedation, intubation, and mechanical ventilation with immediate transfer to the intensive care unit. One day later, he developed convulsive status epilepticus with repeated episodes of generalized tonic-clonic seizures. He was administered phenytoin and valproic acid and maintained on maintenance therapy with three antiepileptic drugs (phenytoin, valproic acid, and levetiracetam). His electroencephalogram (EEG) showed generalized slowing of the background with frequent generalized epileptiform discharges. Despite treatment, the patient remained in refractory status epilepticus, after which he was fully sedated with midazolam. On the third day, MRI of the brain showed innumerable deep-white-matter, high-signal foci on T2-weighted images and low-signal foci on T1-weighted images showing restricted diffusion on diffusion-weighted imaging. Some foci showed blooming artifacts on SWI, consistent with multiple small infarctions, and some foci showed hemorrhagic components consistent with fat embolism (Fig. 3). The patient continued to have refractory generalized tonic-clonic seizures, and workups for other differential diagnoses, including central nervous system infection and autoimmune encephalitis, were negative. He eventually improved and was transferred to the orthopedic ward, where he was managed conservatively. The patient was subsequently discharged and received multidisciplinary care, including rehabilitation.
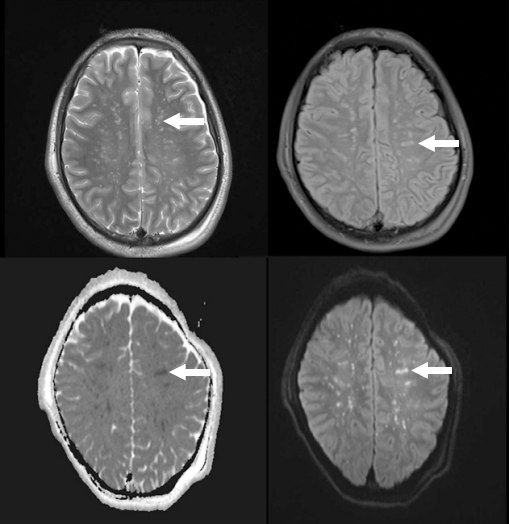
Brain magnetic resonance images show innumerable deep-white-matter, high-signal foci on T2-weighted images and low-signal foci on T1-weighted images showing restricted diffusion on diffusion-weighted imaging. Some images show blooming artifacts on susceptibility-weighted imaging consistent with multiple small infarctions and some show hemorrhagic components consistent with fat embolism. Arrows indicate one of the foci as an example.
Discussion
Fat embolism syndrome is characterized by the classic triad of respiratory compromise, neurological impairment, and petechial skin rashes [4]. Neurological manifestations usually occur 12 to 72 hours after the initial insult. These neurological complications include cerebral infarction, spinal cord ischemia, hemorrhagic stroke, seizures, and coma. Other features include an acute confusional state, autonomic dysfunction, and retinal ischemia. These neurological symptoms and signs are usually nonlocalized with variable severity, which may present a diagnostic challenge, especially in the absence of simultaneous pulmonary and/or dermatological manifestations [5]. None of our patients had a petechial skin rash; one patient experienced oxygen desaturation but showed no radiographic lung changes. The final diagnosis was established mainly by classical neuroimaging findings and exclusion of mimickers such as brain hemorrhage or ischemia following head trauma, metabolic encephalopathy, and drug toxicity. MRI of the brain is the diagnostic modality of choice for cerebral fat embolism syndrome.
Neurological involvement occurs as a result of the migration of fat globules to the central nervous system following adipose tissue damage and the passage of fat globules into open-ended ruptured venous channels. This physical mechanism is augmented by additional biochemical mechanisms incorporating plasma intermediaries that promote fat embolization from storage, with subsequent formation of fat globules in the intravascular compartment. Severe hypoxia due to respiratory failure is another contributing factor [6].
Although neurological manifestations may occur in up to 65% of patients with fat embolism syndrome, seizures occur in only 20% of cases [7]. Seizures could be focal, with or without secondary generalization, or generalized tonic-clonic seizures from the onset. There have been only three previous reports of nonconvulsive status epilepticus secondary to fat embolism [8]. The third patient in our series appears to be the first case of super-refractory convulsive status epilepticus secondary to cerebral fat embolism reported in the literature. Seizures and status epilepticus from cerebral fat embolism are treated in the same manner as those from any other cause, utilizing the available protocols practiced in the treatment center. EEG, especially continuous EEG monitoring, is a necessary tool for the diagnosis, response to antiepileptic therapy, and detection of nonconvulsive status epilepticus.
MRI is the most sensitive tool for demonstrating changes in the brain related to fat embolism syndrome. Most lesions are distributed in specific areas of the brain, including the centrum semiovale, subcortical white matter, ganglionic regions, and thalamus. In addition, five distinct brain patterns have been reported in the literature, namely starfield-scattered cytotoxic edema, confluent cytotoxic edema in white matter, vasogenic edema lesions that may enhance, petechial hemorrhage of white matter, and chronic sequelae patterns [9]. The neuroimages in our case were fascinating; the diagnoses were suspected and confirmed based on these images. We recommend performing MRI of the brain in any patient with long-bone fractures who develops unexplained deterioration in the level of consciousness and seizures.
The mainstay treatment for fat embolism syndrome is preventive and supportive measures with no specific treatment guidelines. The use of steroids is controversial, although the hypothetical benefit may be justified by their anti-inflammatory role and capacity to reduce capillary permeability [10]. Based on observations from the patients reported in our case series, early and adequate hemodynamic and respiratory support and prevention of sepsis are major steps to avoid morbidity and mortality. In addition, early surgical intervention performed within 24 hours after trauma may reduce the risk of cerebral fat embolism.
Cerebral fat embolism is a rare syndrome that is potentially devastating and has poorly elucidated pathophysiological mechanisms. It usually occurs as a complication of long-bone fractures and joint reconstruction surgery. Cerebral fat embolism may occur without any respiratory or dermatological signs. In these cases, diagnosis is established after excluding other differential diagnoses. Neuroimaging using brain MRI is of paramount importance in establishing a diagnosis. Early aggressive hemodynamic and respiratory support and consideration of orthopedic surgical intervention within the first 24 hours after trauma are critical to decreasing morbidity and mortality.
Notes
Conflicts of interest
No potential conflicts of interest relevant to this article was reported.
Funding
None.
Author contributions
Conceptualization: HAA, SAA; Data curation: HAA, NA, FA, WA; Formal analysis: HAA, BHS, NA, FA, WA; Validation, Resources, Supervision, Project administration: HAA; Visualization: HAA, WA; Writing-original draft: all authors; Writing-review & editing: all authors.
