Regenerative potential of biphasic calcium phosphate and enamel matrix derivatives in the treatment of isolated interproximal intrabony defects: a randomized controlled trial
Article information
Abstract
Background
The combined use of biomaterials for regeneration may have great biological relevance. This study aimed to compare the regenerative potential of biphasic calcium phosphate (BCP) alone and with growth factor enamel matrix derivatives (EMDs) for the regeneration of intrabony defects at 1 year.
Methods
This randomized controlled trial included 40 sites in 29 patients with stage II/III periodontitis and 2/3 wall intrabony defects that were treated with BCP alone (control group) or a combination of BCP and EMD (test group). BCP alloplastic bone grafts provide better bio-absorbability and accelerate bone formation. EMDs are commercially available amelogenins. Mean values and standard deviations were calculated for the following parameters: plaque index (PI), papillary bleeding index (PBI), vertical probing pocket depth (V-PPD), vertical clinical attachment level (V-CAL), and radiographic defect depth (RDD). Student paired and unpaired t-tests were used to compare the data from baseline to 12 months for each group and between the groups, respectively. The results were considered statistically significant at p<0.05.
Results
At 12 months, the PI and PBI scores of the control and test groups were not significantly different (p>0.05). The mean V-PPD difference, V-CAL gain, and RDD difference were statistically significant in both groups at 12 months (p<0.001 for all parameters). Intergroup comparisons showed that the mean V-PPD reduction (2.13±1.35 mm), V-CAL gain (2.53±1.2 mm), and RDD fill (1.33±1.0 mm) were statistically significant between the groups at 12 months (p<0.001 for all parameters).
Conclusion
BCP and EMDs combination is a promising modality for the regeneration of intrabony defects.
Introduction
The goal of periodontal therapy is to arrest destructive disease and simultaneously reconstruct lost tissue to maintain health and function. Regeneration of intrabony defects has been attempted using various therapeutic modalities such as bone substitutes, guided regenerative approaches, and tissue arbitrators, including enamel matrix proteins and various growth factors [1]. Evidence from past animal models and in vivo clinicohistologic studies indicates that guided tissue regeneration (GTR) permits the regeneration of new periodontal attachments. Nevertheless, the conventional GTR technique still has unresolved problems. Limited or unpredictable regeneration, early membrane exposure, delayed healing, and the need for technique-sensitive surgical skills are examples of issues faced by many clinicians [2,3].
When selecting a bone substitute for regenerative therapy, autologous bone grafts appear to be the gold standard in terms of anticipated regeneration [4]. Nevertheless, a donor surgical area is required to procure endogenous bone, increasing morbidity. Alternatively, allografts and xenografts have been documented as bone replacement grafts. However, partial resorption and the risk of disease transmission are a few issues reported with these biomaterials [4]. In contrast, synthetic, inorganic, biocompatible alloplastic bone grafts, which have advantages of trouble-free accessibility, no requirement for donor tissue, and no risks of disease transmission, are showing optimistic outcomes as substitutes. Alloplastic biomaterials, such as hydroxyapatite (HA), bioactive glass, calcium sulfate, and calcium phosphates have been utilized as bone substitutes by many clinicians [5,6]. Among these, calcium phosphate-based materials such as HA and β-tricalcium phosphate (β-TCP) have been validated as their structural framework closely mimics the inorganic structure of bone. Controlled clinical trials conducted by Döri et al. [7] in 2005 and Kim et al. [8] in 2010 showed significant bone gain using HA and β-TCP materials. The HA/β-TCP combination is a relatively new biomaterial termed biphasic calcium phosphate (BCP). It is a mixture of HA (60%) in fully crystalline form and particulate β-TCP (40%), which provides better bio-absorbability and accelerates bone formation.
Growth factors are polypeptide hormones known to amalgamate the extracellular matrix, increase proliferation, and promote the migration of periodontal regenerative cells. Growth factors also help differentiate cementoblasts and osteoblasts; therefore, they are an inherent aid in regeneration [2]. Recent in vivo and animal model research has revealed that Hertwig’s epithelial root sheath (HERS) cells release amelogenins [9]. These amelogenin proteins deposited on the root surface correlate with the inception of acellular cementum. Amelogenins are now commercially available as enamel matrix derivatives (EMDs) and are utilized as a component of regenerative therapy. Previous studies conducted by Heijl et al. [10] and other researchers have shown that EMD application in intrabony defects may guide significantly more improvements in attachment and bone fill. The clinically integrated application of EMDs and BCP in intrabony defects might be of great biological relevance. Osteoconductive BCP provides soft tissue support during the initial healing phase while maintaining the space crucial for regeneration [11]. Jensen et al. [12] speculated that the slow bio-absorbable properties of BCP grafts might provide adequate time for EMD to enhance its effect. BCP also acts as a space maintainer, ultimately enhancing the desired outcome.
To the best of our knowledge, there is a research gap and limited available literature concerning the clinical outcomes achieved following the combined use of EMD and BCP. Therefore, this in vivo, prospective, randomized controlled clinical study aimed to compare the clinical and radiographic outcomes obtained by the combined use of BCP and EMD with alloplastic BCP grafts alone in the treatment of periodontal two- or three-wall intrabony defects.
Methods
Ethical statements: The research protocol was approved by the Institutional Review Board (IRB) of Pacific Dental College and Hospital, PAHER University (IRB No: PDCH/21/EC-289). All those willing to participate in this study were provided with a copy of the research protocol and signed informed consent was obtained. The patients were thoroughly informed about the benefits, possible outcomes, and risk factors associated with the treatment/investigation. This study was conducted according to the Helsinki Declaration of 1975, as revised in 2014 [13].
1. Study design
The study was an in vivo randomized controlled clinical trial. A total of 40 sites with two- or three-wall interproximal intrabony defects were identified in 29 patients from the hospital’s outpatient department who were suffering from stage II/III periodontitis requiring regenerative periodontal surgery. The study was carried out following the CONSORT (Consolidated Standards of Reporting Trials) statement (http://www.consortstatement.org/). The 40 defect sites were randomly and equally allocated into two groups using computer-generated numbers: the group treated with BCP (GoldOss, Roseville, MI, USA) alone (control group) and Group B treated with BCP and EMD (Straumann Emdogain, Basel, Switzerland) (test group).
1) Inclusion criteria
The inclusion criteria were as follows: diagnosed with periodontitis [14] (stage II or III), free of any systemic disease, not receiving medications that could alter the surgical results, non-smokers, non-tobacco chewers, aged ≥18 years, having an optimal level of oral hygiene (plaque index [PI] score of <1) (Turesky-Gilmore-Glickman Modification of Quigley-Hein) [15], compliant with the maintenance program, having at least one interproximal intrabony defect (two- or three-wall defect) with a probing depth of ≥6 mm, having an isolated intrabony defect of ≥3 mm as detected on radiography, and having a vital pulp response to electric pulp testing.
2) Exclusion criteria
The exclusion criteria were as follows: noncompliant with a periodontal maintenance program, smokers (≥10 cigarettes per day), tobacco chewers, exhibiting >grade 1 mobility of teeth in the treatment area, history of previous periodontal surgery, and pregnant and lactating women.
2. Interventions
1) Pretreatment
Initially, phase I therapy consisted of scaling, root planing, and oral hygiene instructions; occlusal adjustment was carried out as needed. Six weeks after the initial therapy, the patients were thoroughly evaluated for their plaque control level and need for planned periodontal surgery.
2) Surgical procedure: flap design
The surgical protocol emphasized complete asepsis and infection control. Presurgical rinsing with 0.2% chlorhexidine gluconate (Clohex ADS, Dr. Reddy’s Laboratories Ltd., Hyderabad, India) for 1 minute was performed, followed by injection of local anesthesia (2% lignocaine:epinephrine, 1:100,000). The flap design began with an intrasulcular incision using Bard-Parker (Matronix India Corp., New Delhi, India) no. 15 surgical blades on the buccal and lingual/palatal aspects. The incisions were continued interproximally as far as possible to preserve the entire interdental papilla and achieve primary wound closure. A full-thickness mucoperiosteal envelope flap was carefully reflected facially and lingually using a periosteal elevator (Hu-Friedy, Chicago, IL, USA) to expose the alveolar bone margin. The exposed intrabony osseous defect was debrided of granulation tissue using hand curettes (Hu-Friedy) and ultrasonic instruments with copious saline irrigation. Any granulomatous tissue that adhered to the inner surface of the flap was carefully removed. The root surfaces were plained until a smooth, hard consistency was obtained. Osseous defects were measured vertically at their deepest point from the osseous crest. The flap design was the same for both the control and test groups.
3) Placement of graft/biomaterials
At the control site (total of 20), the required quantity of BCP (synthetic nanocrystalline HA and β-TCP composite) mixed with normal saline solution was placed incrementally and packed. The particle size of the bone graft was 600 to 700 μm (Fig. 1A). At the test sites, the exposed root surface was conditioned with ethylenediaminetetraacetic acid gel (24% ethylenediaminetetraacetic acid gel, pH 6.7; Prefgel, Straumann, Basel, Switzerland) for 2 minutes to remove the smear layer. The root was then thoroughly rinsed with saline and excess fluid was removed, ensuring no blood or saliva contaminating the root surfaces. EMD was then applied immediately, starting at the most apical end of the defect and covering the entire denuded root surface (Fig. 2A). Next, the combination of EMD and BCP was gently packed into the defect and filled to the most coronal level of the defect walls (Fig. 2B).
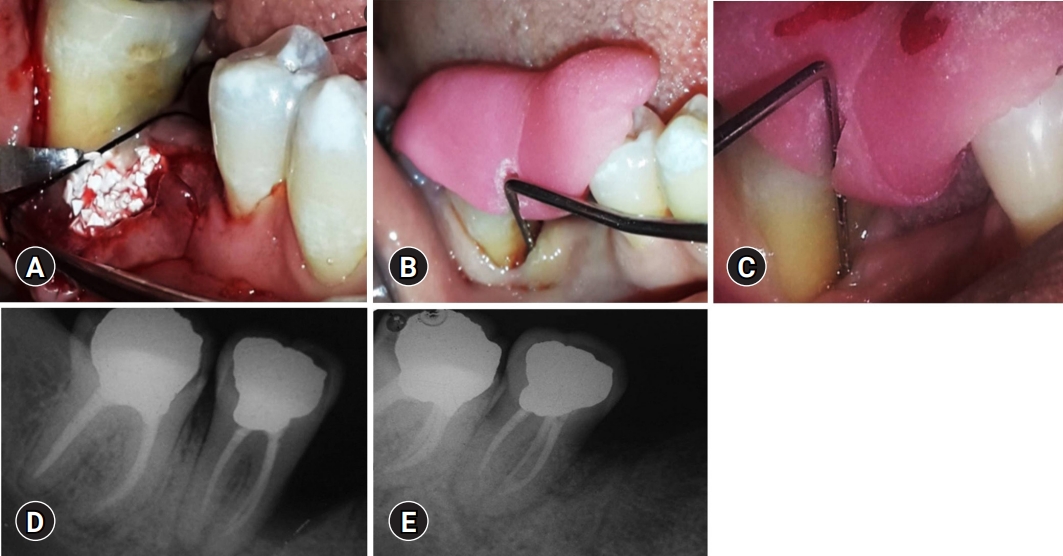
Treatment of the control group. (A) Placement of biphasic calcium phosphate bone graft after debridement of the bone defect in the control group sites. (B) Baseline measurement of V-PPD and V-CAL in the control group. (C) Twelve-month postoperative measurement of V-PPD and V-CAL in the control group. (D) Baseline measurement of RDD in the control group. (E) Twelve-month postoperative measurement of RDD in the control group. V-PPD, vertical probing pocket depth; V-CAL, vertical clinical attachment level; RDD, radiographic defect depth.
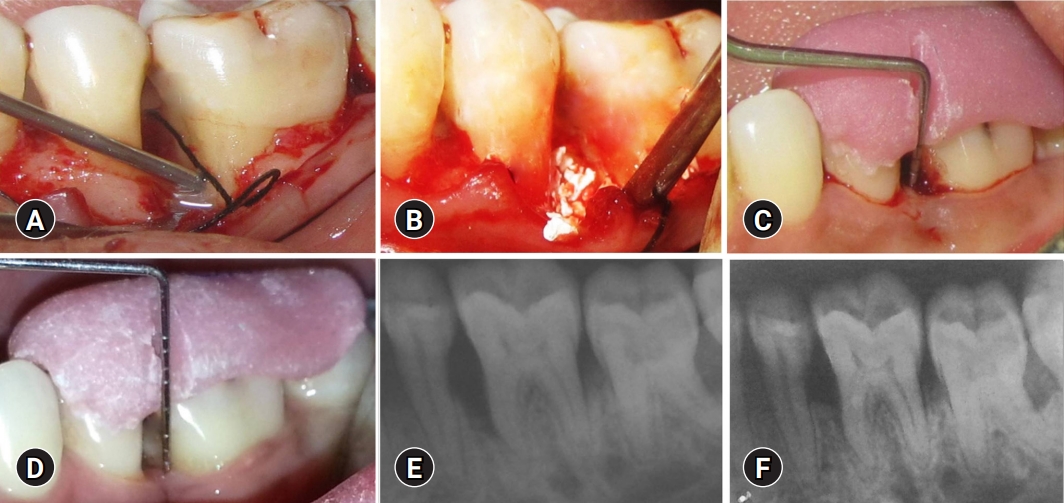
Treatment of the test group. (A) Application of enamel matrix derivative to the defect site in the test group. (B) Placement of biphasic calcium phosphate bone graft and enamel matrix derivative after debridement of the bone defect in the test group sites. (C) Baseline measurement of V-PPD and V-CAL in the test group. (D) Twelve-month postoperative measurement of V-PPD and V-CAL in the test group. (E) Baseline measurement of RDD in the test group. (F) Twelve-month postoperative measurement of RDD in the test group. V-PPD, vertical probing pocket depth; V-CAL, vertical clinical attachment level; RDD, radiographic defect depth.
4) Suturing
The mucoperiosteal flap was repositioned and suturing was performed using 3-0 nonabsorbable silk sutures (Ethicon Mersilk, Johnson & Johnson Ltd., Raritan, NJ, USA). Periodontal dressings (COE-PAK, GC America Inc., Alsip, IL, USA) were used to cover the surgical wounds. Seven days postoperatively, the periodontal dressings and sutures were removed.
3. Postoperative care
The patients were prescribed nonsteroidal anti-inflammatory drugs (IBUGESIC, ibuprofen+paracetamol; one tablet, three times per day for 5 days). The patients were instructed not to brush the treated sites for 1 week. A 0.2% chlorhexidine gluconate mouth rinse (Clohex ADS), twice daily for 1 minute was prescribed for 4 weeks to maintain optimal oral hygiene. Recall appointments were scheduled every 14 days during the first 3 months following surgical procedures and every 3 months thereafter until the study period ended to reinforce the oral hygiene instructions and provide supragingival ultrasonic scaling if required. The participants were reevaluated for statistical analysis 12 months after surgical therapy. All clinical parameters were recorded, and clinical intraoral photographs were obtained preoperatively and postoperatively at 12 months. A blinded second clinician evaluated all parameters, unaware of the specific treatment group recruitment.
4. Outcome measures
The primary outcome variables of the study were the vertical clinical attachment level (V-CAL), vertical probing pocket depth (V-PPD), and radiographic defect depth (RDD). The secondary outcomes were the PI score (Turesky-Gilmore-Glickman Modification of Quigley-Hein) [15] and papillary bleeding index (PBI) score [16].
1) Clinical measurements
Grooved occlusal stents were fabricated using acrylic resin to maintain standardization and to guide periodontal probe insertion. The V-CAL was measured using a graduated Williams periodontal probe from the apical border of the acrylic stent to the base of the pocket. The V-PPD distance from the apical border of the occlusal stent to the gingival margin was measured, and the measured length was subtracted from the V-CAL distance (Fig. 1B, 1C, 2C, 2D).
2) Radiographic measurements
Intraoral periapical radiographs were obtained using the long-cone paralleling technique and digitized using Film Scan 75 NDT interface software (Shield Alloys India Pvt. Ltd., Mumbai, India). The RDD was measured on the radiograph as follows: RDD at baseline, most coronal point of the alveolar crest to the base of the bone defect as distinguished on the radiograph (Fig. 1D, 2E); RDD at 12 months, most coronal point of the alveolar crest to the base of the bone defect 12 months after intervention as distinguished on the radiograph (Fig. 1E, 2F).
5. Statistical analysis
Statistical analysis was performed using a statistical software package (PASS software, NCSS, Kaysville, UT, USA), and each patient was considered a statistical unit. Means and standard deviations were calculated for the PI, PBI, V-PPD, V-CAL, and RDD. The Student paired t-test was used to compare the data from baseline to 12 months for each group, and the Student unpaired t-test was used between groups at 12 months follow-up. The results were considered nonsignificant, significant, highly significant, and very highly significant when p>0.05, p<0.05, p<0.001, and p<0.0001, respectively.
Results
Twenty-nine participants (16, male and 13, female; mean age, 33.7±3.1 years) with 40 intrabony defect sites present on their mandibular premolars or molars were recruited for the study. After 12 months, all patients completed the trial and all 40 sites (n=40, 20 tests and 20 controls) had been analyzed.
1. Plaque index score (n=40)
In the control group at baseline, the mean PI score was 0.77±0.08 and at 12 months, it was 0.79±0.12 (mean difference, 0.02±0.09), which was statistically nonsignificant (p=0.326). In the test group at baseline, the mean PI score was 0.74±0.05 and at 12 months, it was 0.72±0.11 (mean difference, 0.02±0.07), which was also statistically nonsignificant (p=0.259) (Table 1).
2. Papillary bleeding index score (n=40)
In the control group at baseline, the mean PBI score was 0.78±0.091 and at 12 months, it was 0.75±0.092 (mean difference, 0.03±0.01), which was statistically nonsignificant (p=0.281). In the test group at baseline, the mean PBI score was 0.77±0.091 and at 12 months, it was 0.75±0.091 (mean difference, 0.07±0.01), which was also statistically nonsignificant (p=0.422) (Table 1).
3. Vertical probing pocket depth (n=40)
In the control group, the mean V-PPD at baseline and at 12 months was 7.27±0.07 mm and 4.40±0.91 mm, respectively. The mean V-PPD difference of 2.87±0.83 mm was highly significant (p<0.001). In the test group, the mean V-PPD at baseline and at 12 months was 7.53±0.74 mm and 2.53±0.74 mm, respectively. The mean V-PPD reduction of 5.00±1.31 mm at 12 months was highly significant (p<0.001) (Tables 2, 3). When comparing the difference in V-PPD reduction at 12 months between the test and control groups, it was 2.13±1.35 mm, which was also highly significant (p<0.001) (Table 4).
4. Vertical clinical attachment level (n=40)
In the control group, the mean V-CAL at baseline and 12 months was 10.33±0.72 mm and 7.73±0.96 mm, respectively. The mean V-CAL gain of 2.60±0.63 mm was highly significant (p<0.001). In the test group, the mean V-CAL at baseline and 12 months was 10.47±0.74 mm and 5.33±0.82 mm, respectively. The mean V-CAL gain of 5.13±0.92 mm was highly significant (p=0.001) (Tables 2, 3). When comparing the difference in V-CAL gain at 12 months between the test and control groups, it was 2.53±1.2 mm, which was also highly significant (p<0.001) (Table 4).
5. Radiographic defect depth (n=40)
In the control group, the mean RDD at baseline was 3.47±0.52 mm, which was reduced to 2.07±0.59 at 12 months. The mean difference of 1.40±0.51 mm was highly significant (p<0.001). The RDD at baseline and 12 months in the test group was 3.73±0.70 mm and 1.00±0.00 mm, respectively. The mean difference of 2.73±0.70 mm was highly significant (p<0.001) (Tables 2, 3). The mean RDD difference between the test and control groups at 12 months was 1.33±1.0 mm, which was also highly significant (p<0.001) (Table 4).
Discussion
The main objective of this study was to compare the regenerative potential of the BCP-EMD combination with BCP alone. All 29 participants maintained good oral hygiene levels throughout the study, as indicated by the PI and PBI scores. Both treatment groups showed statistically significant V-PPD reductions, V-CAL gains, and RDD fills at 1 year. Intergroup comparisons of the primary outcome parameters showed statistically significant differences. None of the subjects experienced any adverse reactions. BCP and EMD did not cause any allergic or foreign body reactions. A crucial clinical parameter for validating regeneration is the gain in V-CAL. The mean V-CAL gain in the control group observed in the present study is comparable to the results of the studies published by Shetty and Bose [17] (2.0±0.58 mm) in 2013, Lee et al. [18] (3.0±1.1 mm) in 2012, and Stein et al. [19] (3.1±0.8 mm) in 2009.
Past research conducted by Ozdemir and Okte [20] in 2012 showed that intrabony defects (n=14) treated with BCP had a statistically significant V-CAL gain (p=0.002) at 6 months. Similarly, the mean V-CAL gain in the test group of the current study was comparable with the values found by Pietruska et al. [21] (12 intrabony defects, V-CAL gain of 3.6±0.1 mm at 1 year), Francetti et al. [22] (3.41±0.14 mm), Parodi et al. [23] (3.08±1.45 mm), and Sculean et al. [24] (10 intrabony defects, CAL gain of 3.0±0.3 mm at 9 months). In the present study, the difference in mean V-CAL gain between the test and control groups (2.53±1.2 mm) at 12 months was highly significant (p<0.001).
In the present study, highly significant reductions in V-PPD were observed in both the control (2.87±0.8 mm) and test (5.0±1.31 mm) groups. Overall, the test group showed a more significant V-PPD reduction than did the control group at 12 months. The V-PPD reduction in the control group is similar to the values found in the studies reported by Kaushick et al. [25], Pandit et al. [26], and Lee et al. [18], and the V-PPD of the test group is equivalent to the value found in the study conducted by Pietruska et al. [21]. In a meta-analysis in 2021, Jasser et al. [27] compared the effectiveness of BCP with other bone substitute materials in periodontal infrabony defects and found that the defect regeneration with BCP was superior to that with debridement alone. BCP also showed comparable results to other bone graft materials, such as frozen allogeneic grafts and HA cement granules, in terms of V-PPD reduction, CAL gain, or bone fill. The same meta-analysis also revealed that regeneration of periodontal infrabony defects using BCP in combination with growth factors resulted in poor outcomes. A systemic review conducted by Dewi and Ana in 2018 showed that the combined use of HA and β-TCP significantly improved regeneration [28]. In a 2016 systematic review evaluating growth factors and BCP with autogenous or allogeneic grafts for periodontal intraosseous defects, Cãlin and Pãtraşcu [29] found that BCP had a comparable V-PPD reduction, V-CAL gain, and bone fill. A systematic review conducted by Stavropoulos et al. [30] in 2022 reported the outcomes of grafting, GTRs, EMDs, and various combinations. They found that most reported studies used GTRs or EMDs. GTRs were typically performed using resorbable membranes. The bone substitute materials reviewed were alloplastic (11 groups) and xenografts (eight groups), whereas five groups used combined therapy. Combination approaches were found to be more efficacious, such as the use of a bone graft/substitute with EMDs or other growth factors [30]. The literature shows that almost all current bone graft materials primarily serve as a structural framework for osteoregenerative processes to occur; thus, they only satisfy the osteoconductive component.
Although a few researchers have used only EMDs in intrabony defects, EMDs have viscous rheological properties, which may not be sufficient to prevent flap collapse into the desired regeneration area. Hence, the use of a BCP may help in space maintenance during the regeneration period. Vandana et al. [31] used granulated HA in eight intrabony defects, resulting in a V-CAL gain of 1.75±0.46 mm at 9 months. Okuda et al. [32] used HA in 35 intrabony defects and found a CAL gain of 2.0±1.2 mm at 12 months. In our present study, a CAL gain of 2.60±0.63 mm was found at 12 months in the BCP group, which is higher than that observed with HA in the aforementioned studies.
Animal model studies have indicated that BCP (60% HA+40% β-TCP in particulate form) allows for better control of the resorbability of the graft material and accelerates de novo bone formation. Hence, BCP provides better results than HA or β-TCP grafts individually [33]. Lynch et al. [34] reported that osteoconductive BCP acts as a scaffold for bone maturation and initiates differentiation. Gestrelius et al. [35] hypothesized that a sufficient retention time of ceramic material (BCP in the case of our study) in the defect area acts as a barrier to the apical migration of the dentogingival epithelium. Additionally, EMD creates a synergistic effect to prevent apical migration of the epithelium. Gestrelius et al. [35] also reported that EMD retarded epithelial growth into intrabony defects. Van der Pauw et al. [36] reported that EMDs have a stimulating effect on periodontal attachment and fibroblasts on the cemental surface during the early stages of wound healing. EMDs also stimulate the expression of alkaline phosphatase, which may increase the cementogenic capacity of human periodontal ligament fibroblasts (HPLFs). Zetterström et al. [37] reported that EMD stimulates the release of transforming growth factor β1 (TGF-β1) by HPLFs and HGFs. Ellegaard and Löe [38] studied EMD-integrated regeneration of treated intrabony defects by selective cell repopulation, which may influence the fibroblast migration rate and TGF-β1 production. A 12-month surgical reentry study of intrabony defects showed that EMD stimulated the proliferation of pre-osteoblasts and differentiation of immature osteoblasts [38].
Radiographic evaluation of bone changes following regenerative therapy is a noninvasive alternative to surgical reentry. In the present study, both the control and test groups showed significantly improved linear bone filling at 12 months. In addition, the test group showed higher bone fill than the control group, which was highly significant. This result of the defect fill in the control group was in agreement with the results reported by Lee et al. [18] and Shetty and Bose [17]. Meyle et al. [39], in a multicenter randomized controlled trial, treated one- or two-wall intrabony defects with EMD and synthetic bone grafts. The combination treatment group showed 2.7±1.9 mm of bone fill and 1.7±2.1 mm of V-CAL gain at 1-year follow-up, which is comparable with the results of the present study. In our study, among the test group defects, 10 sites gained 80%, five defects gained 60%, and five sites gained 40% bone fill. Among the control group defects, 12 gained 40% to 60% bone fill, and eight gained <40% bone fill. Osseous defect fill is multifactorial and depends on the graft biomaterial, type and morphology of the defect, and surgical skills. Ellegaard and Löe [38] reported that three-wall intrabony defects have a higher chance of bone fill than two- or one-wall defects. Schallhorn et al. [40] reported that the degree of bone fill is associated with the morphology of intrabony defects. In our study, three- or two-wall defects were included in both the control and test groups. One limitation of the present study is the impracticality of measuring bone gain using surgical reentry or histological evidence of regeneration for ethical reasons. The present study showed that at 12 months, BCP and EMD combination therapy resulted in a statistically significant improvement in pocket depth reduction, clinical attachment gain, and radiographic bone fill compared with that of BCP alone. Furthermore, the literature showed that regenerative outcomes of BCP can be enhanced when combined with growth factors such as EMDs to achieve results similar to those of autografts or allografts. The obtained data also indicate the effectiveness and safety of BCP or EMD applications.
Clinicians should consider BCP and EMD combination as a realistic, predictable, and practical regenerative modality for regeneration in deep intrabony defects. It is a promising alternative approach in situations where autogenous/allogeneic or xenografts cannot be used because of unavailability, ethical reasons, or cost issues. Long-term maintenance and histological evidence of bone fill must be thoroughly established before these approaches are used in lieu of established prognostic techniques.
Notes
Conflicts of interest
No potential conflicts of interest relevant to this article was reported.
Funding
None.
Author contributions
Conceptualization: All authors; Investigation: PCP, AB, SS, KT, SK; Data curation: PCP, AB, RB, SS, SK; Methodology: PCP, AB, SS, SK; Formal analysis: AB, RB; Validation: PCP, SK; Project administration: PCP, RB; Resources: RB, SK; Software: AB, SS, SK; Visualization: PCP, RB, KT; Supervision: AB; Writing-original draft: PCP, AB, RB, KT; Writing-review & editing: AB, SS, KT, SK.




