PubMed Central, CAS, DOAJ, KCI

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Volume 40(3); 2023 > Article
-
Case report
Severe congenital neutropenia mimicking chronic idiopathic neutropenia: a case report -
Juhyung Kim1
 , Soyoon Hwang2
, Soyoon Hwang2 , Narae Hwang3,4
, Narae Hwang3,4 , Yeonji Lee5
, Yeonji Lee5 , Hee Jeong Cho1
, Hee Jeong Cho1 , Joon Ho Moon6
, Joon Ho Moon6 , Sang Kyun Sohn1
, Sang Kyun Sohn1 , Dong Won Baek6
, Dong Won Baek6
-
Journal of Yeungnam Medical Science 2023;40(3):283-288.
DOI: https://doi.org/10.12701/jyms.2022.00353
Published online: July 28, 2022
1Department of Hematology/Oncology, Kyungpook National University Hospital, School of Medicine, Kyungpook National University, Daegu, Korea
2Department of Infectious Diseases, Kyungpook National University Chilgok Hospital, School of Medicine, Kyungpook National University, Daegu, Korea
3Department of Clinical Pathology, School of Medicine, Kyungpook National University, Daegu, Korea
4Department of Laboratory Medicine, Kyungpook National University Chilgok Hospital, Daegu, Korea
5Department of Internal Medicine, Kyungpook National University Hospital, Daegu, Korea
6Department of Hematology/Oncology, Kyungpook National University Chilgok Hospital, School of Medicine, Kyungpook National University, Daegu, Korea
- Corresponding author: Dong Won Baek, MD, PhD Department of Hematology/Oncology, Kyungpook National University Chilgok Hospital, School of Medicine, Kyungpook National University, 807 Hogukno, Buk-gu, Daegu 41404, Korea Tel: +82-53-200-2623 • Fax: +82-53-200-2029 • E-mail: baekdw83@gmail.com
Copyright © 2023 Yeungnam University College of Medicine, Yeungnam University Institute of Medical Science
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,297 Views
- 123 Download
Abstract
- Severe chronic neutropenia is classified as severe congenital, cyclic, autoimmune, or idiopathic. However, there is a lot of uncertainty regarding the diagnosis of severe congenital neutropenia (SCN) and chronic idiopathic neutropenia, and this uncertainty affects further evaluations and treatments. A 20-year-old man presented with fever and knee abrasions after a bicycle accident. On admission, his initial absolute neutrophil count (ANC) was 30/µL. He had no medical history of persistent severe neutropenia with periodic oscillation of ANC. Although his fever resolved after appropriate antibiotic therapy, ANC remained at 80/µL. Bone marrow (BM) aspiration and biopsy were performed, and a BM smear showed myeloid maturation arrest. Moreover, genetic mutation test results showed a heterozygous missense variant in exon 4 of the neutrophil elastase ELANE: c597+1G>C (pV190-F199del). The patient was diagnosed with SCN. After discharge, we routinely checked his ANC level and monitored any signs of infection with minimum use of granulocyte colony-stimulating factor (G-CSF), considering its potential risk of leukemic transformation. Considering that SCN can be fatal, timely diagnosis and appropriate management with G-CSF are essential. We report the case of a patient with SCN caused by ELANE mutation who had atypical clinical manifestations. For a more accurate diagnosis and treatment of severe chronic neutropenia, further studies are needed to elucidate the various clinical features of ELANE.
- Neutropenia is a hematological condition characterized by a reduced absolute neutrophil count (ANC) of <1,500/µL [1]. Severe neutropenia with an ANC of <500/µL increases susceptibility to serious bacterial or fungal infections [2]. Severe chronic neutropenia that lasts for >3 months is categorized as severe congenital, cyclic, autoimmune, or idiopathic neutropenia [1-3]. Severe congenital neutropenia (SCN) occurs in only two cases per million people, whereas cyclic neutropenia (CyN) occurs in 0.6 cases per million people [4]. Chronic idiopathic neutropenia (CIN) and autoimmune neutropenia (AIN) are also rare diseases. The prevalence of CIN is approximately 1 to 2 cases per million people among children and adults [2,5].
- Genetic mutation is the most important feature that distinguishes congenital and CyN from other types of neutropenia [4]. ELANE (NM_001972) gene mutations are observed in 40% to 60% of SCN cases and in 80% to 100% of CyN cases [6]. CyN is characterized by regular oscillations in peripheral blood neutrophils with a 21-day periodicity, whereas SCN is a more severe disease with a consistently low ANC of <200/µL. CIN is characterized by a normal bone marrow (BM) karyotype without a definable genetic cause, and negative serum anti-neutrophil antibodies [7]. Previous studies have suggested that CIN is caused by immune-mediated BM impairment of neutrophil production, but its etiology has not been fully elucidated [5].
- Granulocyte colony-stimulating factor (G-CSF) is the most effective treatment option for severe chronic neutropenia; however, G-CSF should be used very cautiously [2,5,7]. Leukemic transformation is one of the most frequently reported problems caused by the long-term use of G-CSF, which increases the risk of leukemia, particularly in patients with SCN, but not in patients with CIN [1,3,5]. However, there are not enough data on the pathophysiology of SCN and the genes responsible for it [3]. Moreover, there are many uncertainties regarding the diagnosis of SCN and CIN that affect further evaluation and management. Here, we present a rare case of SCN that mimicked CIN.
Introduction
- Ethical statements: This study was approved by the Institutional Review Board (IRB) of Kyungpook National University Hospital (IRB No: KNUCH 2022-04-038). Written informed consent for publication was obtained from the patient.
- The patient had a history of profound neutropenia with an ANC of <200/µL, with bronchiolitis and asthmatic bronchitis occurring in the first 3 months of life. Whenever this patient arrived at the hospital during his childhood, his initial ANC was >1,500/µL, which decreased to <200/µL during his hospital stay (Fig. 1). Furthermore, his ANC returned to normal after appropriate antibiotic treatment. However, the patient had no regular periodic oscillations in his ANC (Fig. 1). He had a history of oral ulceration and glossitis when he was 13 years old; however, he had no history of parodontopathy, edentulism, or aphthosis. When he was 15 years old, the workup for neutropenia revealed a latent tuberculosis infection (LTBI). After 9 months of isoniazid treatment, the patient was cured of the LTBI. Since 2018, his ANC peak had decreased to <1,300/µL. Nevertheless, he had never undergone BM examination or G-CSF treatment. Although the patient’s mother also had a history of neutropenia, a specific hematologic disorder was not identified in a BM study, and genetic testing, such as DNA sequencing, was not performed.
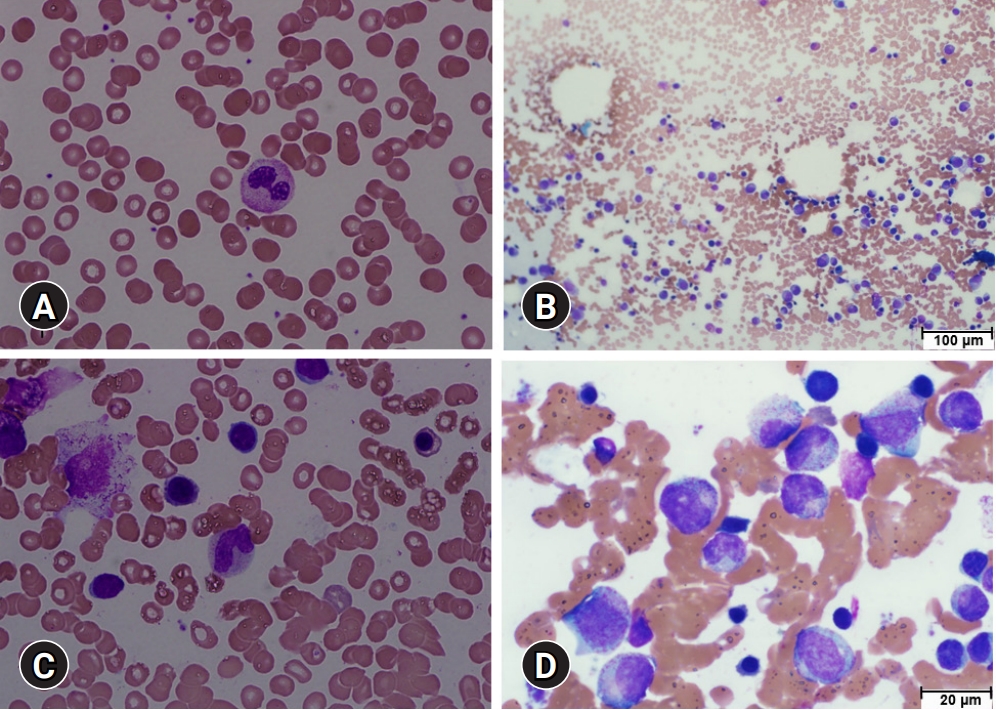
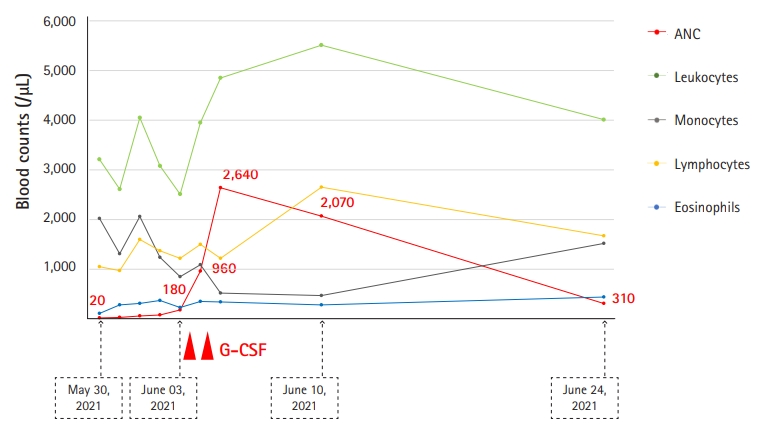
- In May 2021, he was admitted to our hospital with fever and superficial soft tissue injuries caused by a bicycle accident. After admission to our infectious disease department, a physical examination revealed a body temperature of 38°C, blood pressure of 118/72 mmHg, pulse rate of 76 beats/min, and superficial abrasions over the proximal anterior tibia. At the time of admission, his complete blood cell count revealed a white blood cell count of 3,620/µL, ANC of 30/µL, hemoglobin level of 11.7 g/dL, platelet count of 209,000/µL, and serum C-reactive protein (CRP) level of 19.8 mg/dL. However, antinuclear antibody test results were negative. After he received piperacillin/tazobactam (4.5 g every 8 hours) for 3 days, his serum CRP level decreased to 6.2 mg/dL; his fever resolved on the second day of treatment. However, his ANC remained at 80/µL during the first 3 days (Fig. 2). The patient was referred to the hematology department, where BM aspiration and biopsy were performed. A BM smear showed abnormal differentiation of the myeloid lineage (Fig. 3). The granulocytic series showed maturation arrest in the myelocyte stage with few segmented neutrophils (Fig. 3).
- Based on the patient’s medical records, it was reasonable to begin G-CSF pending genetic testing. Lenograstim was administered to the patient at 2 µg/kg/day for 2 days. Although a G-CSF dose of 2–10 µg/kg/day is usually used for patients with SCN, we started with the lowest possible effective dose before confirming the presence of genetic mutations in this patient [8]. His ANC increased to 2,640/µL on the third day with 2 µg/kg/day lenograstim for 2 days. He was discharged from the hospital in a healthy condition after 7 days of treatment for severe neutropenia (Fig. 2). Finally, the genetic mutation test results showed that his severe neutropenia was caused by a heterozygous in-frame deletion in the neutrophil elastase ELANE, with a nucleotide substitution in intron 4 (IVS4+1G>C) located in the consensus splice donor site, resulting in a missense variant in exon 4 of ELANE: c597+1G>C (pV190-F199del) (Table 1, Supplementary Fig. 1) [9]. Therefore, the patient was diagnosed with SCN. Three weeks after discharge, his ANC decreased to <500/µL. Nevertheless, the patient no longer had fever or signs of infection. G-CSF was not routinely administered and was reserved for episodes of infection.
Case
- A nonfunctional, misfolded neutrophil elastase encoded by a mutated ELANE gene accelerates the apoptosis of granulocytic precursors of myeloid cells, causing myeloid maturation arrest [9,10]. ELANE gene mutations are the main cause of SCN and CyN [9]. Some researchers have considered SCN and CyN to represent a continuum with phenotypic variability [10]. It has been suggested that the clinical manifestations of patients with SCN and CyN might be influenced not only by a single genetic factor such as ELANE mutations but also by other genetic or epigenetic factors [11]. Therefore, it is possible to make an accurate initial diagnosis based on an accurate understanding of the pathogenic aspects of congenital neutropenia [12]. SCN is rare but can also be fatal. Furthermore, SCN has various phenotypes that are sometimes atypical, as in the present case.
- Several reports have shown that the ANC is consistently <200/µL in patients with SCN [6]. However, no evidence of constant severe neutropenia (ANC <200/µL) was detected in our patient before 20 years of age. Severe neutropenia followed by recurrent infections is also a typical pattern of SCN and CyN [4]. In contrast, when the patient arrived at a hospital during his childhood, his initial ANC was >1,500/µL, which decreased to <200/µL during his hospital stay; this presentation was incompatible with SCN. Furthermore, the patient did not show regular periodic oscillations in his ANC. Thus, the patient’s nonperiodic mild-to-moderate neutropenia with no evidence of infection and a negative test result for autoimmune disease might have led the previous healthcare workers to consider the diagnosis to be CIN or acquired neutropenia due to infection, and delay a BM examination [8,13-15]. CIN is considered a benign disease and is often self-limiting [2,7]. In young children, approximately 1/3 of CIN cases resolve spontaneously, whereas remission is uncommon in adults [5]. Thus, BM examination should always be considered when chronic neutropenia, which began in early childhood, persists. Considering that the risk of bacterial infection and leukemic transformation in ELANE-related neutropenia is high, precise diagnosis of SCN and early identification are essential for appropriate treatment with G-CSF and further management such as hematopoietic stem cell transplantation (HSCT) [16,17].
- Extensive studies have shown that myeloid proliferation and maturation are stimulated by G-CSF, which can be used to treat severe chronic neutropenia [5,18]. However, since its introduction in 1998, G-CSF has attracted considerable attention owing to its potential risk of malignancy [5,19]. Recently, a strong relationship between G-CSF treatment and malignant transformation in SCN has also been reported [3,17]. In contrast, there is no evidence that G-CSF predisposes leukemic transformation in CIN and AIN [5]. Therefore, the dose, timing, and duration of G-CSF therapy should be very carefully determined in patients with congenital neutropenia, such as SCN and CyN [17,20]. Although we do not consider the long-term use of high-dose G-CSF unless there is sufficient evidence of infection, we do take G-CSF therapy and HSCT into consideration depending on how low the patient’s ANC level falls and the existence of any signs of infection [19]. Here, we report the case of a patient with SCN that mimicked CIN. We hope that patients with atypical features of SCN can be diagnosed in a timely manner and receive appropriate G-CSF treatment. Further studies are needed to elucidate the various clinical phenotypes of ELANE to obtain a more specific and precise diagnosis and treatment of severe chronic neutropenia. Moreover, clinical trials for patients with SCN to establish an appropriate ANC cutoff for G-CSF treatment are warranted to minimize the risk of malignant transformation, depending on the specific ELANE mutation.
Discussion
-
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
-
Funding
None.
-
Author contributions
Conceptualization: DWB, SH; Resources: DWB, SH, JK, YL; Data curation: JK; Visualization: NH; Validation: SKS, JHM, HJC; Writing-original draft: JK; Writing-review & editing: all authors.
Notes
Supplementary materials
Supplementary Fig. 1.
| Location | DNA change | Predicted AA change | Zygosity | OMIM disease | Inheritance | Class |
|---|---|---|---|---|---|---|
| Intron 4 | c.597+1G>C | pV190-F199del | Het | CN, CyN | AD | PV |
- 1. Dale DC, Cottle TE, Fier CJ, Bolyard AA, Bonilla MA, Boxer LA, et al. Severe chronic neutropenia: treatment and follow-up of patients in the Severe Chronic Neutropenia International Registry. Am J Hematol 2003;72:82–93.ArticlePubMed
- 2. Wan C, Yu HH, Lu MY, Lee JH, Wang LC, Lin YT, et al. Clinical manifestations and outcomes of pediatric chronic neutropenia. J Formos Med Assoc 2012;111:220–7.ArticlePubMed
- 3. Donadieu J, Fenneteau O, Beaupain B, Mahlaoui N, Chantelot CB. Congenital neutropenia: diagnosis, molecular bases and patient management. Orphanet J Rare Dis 2011;6:26.ArticlePubMedPMC
- 4. Boxer LA. How to approach neutropenia. Hematology Am Soc Hematol Educ Program 2012;2012:174–82.ArticlePubMedPDF
- 5. Walkovich K, Boxer LA. How to approach neutropenia in childhood. Pediatr Rev 2013;34:173–84.ArticlePubMedPDF
- 6. Dale DC, Bolyard AA. An update on the diagnosis and treatment of chronic idiopathic neutropenia. Curr Opin Hematol 2017;24:46–53.ArticlePubMedPMC
- 7. Newburger PE, Pindyck TN, Zhu Z, Bolyard AA, Aprikyan AA, Dale DC, et al. Cyclic neutropenia and severe congenital neutropenia in patients with a shared ELANE mutation and paternal haplotype: evidence for phenotype determination by modifying genes. Pediatr Blood Cancer 2010;55:314–7.ArticlePubMedPMC
- 8. Sicre de Fontbrune F, Moignet A, Beaupain B, Suarez F, Galicier L, Socié G, et al. Severe chronic primary neutropenia in adults: report on a series of 108 patients. Blood 2015;126:1643–50.ArticlePubMedPDF
- 9. Munshi HG, Montgomery RB. Severe neutropenia: a diagnostic approach. West J Med 2000;172:248–52.ArticlePubMedPMC
- 10. Kurnikova M, Maschan M, Dinova E, Shagina I, Finogenova N, Mamedova E, et al. Four novel ELANE mutations in patients with congenital neutropenia. Pediatr Blood Cancer 2011;57:332–5.ArticlePubMed
- 11. Cho HK, Jeon IS. Different clinical phenotypes in familial severe congenital neutropenia cases with same mutation of the ELANE gene. J Korean Med Sci 2014;29:452–5.ArticlePubMedPMCPDF
- 12. Germeshausen M, Schulze H, Ballmaier M, Zeidler C, Welte K. Mutations in the gene encoding neutrophil elastase (ELA2) are not sufficient to cause the phenotype of congenital neutropenia. Br J Haematol 2001;115:222–4.ArticlePubMedPDF
- 13. Makaryan V, Zeidler C, Bolyard AA, Skokowa J, Rodger E, Kelley ML, et al. The diversity of mutations and clinical outcomes for ELANE-associated neutropenia. Curr Opin Hematol 2015;22:3–11.ArticlePubMedPMC
- 14. Gupta A, Dhingra A. Incidental chronic neutropenia in an asymptomatic adult. Cureus 2017;9:e1779.ArticlePubMedPMC
- 15. Solomou EE, Salamaliki C, Lagadinou M. How to make the right diagnosis in neutropenia. Clin Hematol Int 2021;3:41–6.ArticlePubMedPMC
- 16. Rotulo GA, Beaupain B, Rialland F, Paillard C, Nachit O, Galambrun C, et al. HSCT may lower leukemia risk in ELANE neutropenia: a before-after study from the French Severe Congenital Neutropenia Registry. Bone Marrow Transplant 2020;55:1614–22.ArticlePubMedPMCPDF
- 17. Rotulo GA, Plat G, Beaupain B, Blanche S, Moushous D, Sicre de Fontbrune F, et al. Recurrent bacterial infections, but not fungal infections, characterise patients with ELANE-related neutropenia: a French Severe Chronic Neutropenia Registry study. Br J Haematol 2021;194:908–20.ArticlePubMedPDF
- 18. Dale DC, Bonilla MA, Davis MW, Nakanishi AM, Hammond WP, Kurtzberg J, et al. A randomized controlled phase III trial of recombinant human granulocyte colony-stimulating factor (filgrastim) for treatment of severe chronic neutropenia. Blood 1993;81:2496–502.ArticlePubMedPDF
- 19. Dale DC. The discovery, development and clinical applications of granulocyte colony-stimulating factor. Trans Am Clin Climatol Assoc 1998;109:27–36.PubMedPMC
- 20. Skokowa J, Dale DC, Touw IP, Zeidler C, Welte K. Severe congenital neutropenias. Nat Rev Dis Primers 2017;3:17032.ArticlePubMedPMCPDF
References
Figure & Data
References
Citations


 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine
 PubReader
PubReader ePub Link
ePub Link Cite
Cite