PubMed Central, CAS, DOAJ, KCI

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Volume 38(4); 2021 > Article
-
Review article
An update on immunotherapy with PD-1 and PD-L1 blockade -
Sung Ae Koh

-
Yeungnam University Journal of Medicine 2021;38(4):308-317.
DOI: https://doi.org/10.12701/yujm.2021.01312
Published online: September 9, 2021
Department of Hematology-Oncology, Yeungnam University College of Medicine, Daegu, Korea
- Corresponding author: Sung Ae Koh, MD, PhD Department of Hematology-Oncology, Yeungnam University College of Medicine, 170 Hyeonchung-ro, Nam-gu, Daegu 42415, Korea Tel: +82-53-620-3826 Fax: +82-53-654-8386 E-mail: sakoh@yu.ac.kr
Copyright © 2021 Yeungnam University College of Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 4,137 Views
- 93 Download
- 2 Crossref
Abstract
- Cancer is the leading cause of death and is on the rise worldwide. Until 2010, the development of targeted treatment was mainly focused on the growth mechanisms of cancer. Since then, drugs with mechanisms related to tumor immunity, especially immune checkpoint inhibitors, have proven effective, and most pharmaceutical companies are striving to develop related drugs. Programmed cell death-1 and programmed cell death ligand-1 inhibitors have shown great success in various cancer types. They showed durable and sustainable responses and were approved by the U.S. Food and Drug Administration. However, the response to inhibitors showed low percentages of cancer patients; 15% to 20%. Therefore, combination strategies with immunotherapy and conventional treatments were used to overcome the low response rate. Studies on combination therapy have typically reported improvements in the response rate and efficacy in several cancers, including non-small cell lung cancer, small cell lung cancer, breast cancer, and urogenital cancers. The combination of chemotherapy or targeted agents with immunotherapy is one of the leading pathways for cancer treatment.
- Cancer, the leading cause of death, is on the rise worldwide. According to recently published cancer statistics in Korea in 2018, the growing trend in cancer incidence has slowed compared to 2014, but it can be found that the cancer incidence rate continues to increase until then [1]. Accordingly, there has been a large body of research and progress on gene mutations related to cancer growth and the development of targeted treatments. Until 2010, the development of targeted treatment was mainly focused on cancer’s growth mechanism. However, since then, drugs with mechanisms related to tumor immunity, especially immune checkpoint inhibitors (ICIs), have proven effective [2], and almost all pharmaceutical companies are striving to develop related drugs.
- Tumor immunotherapy refers to cancer treatment using the human immune system. Over the past century, cancer researchers have conducted extensive research to treat cancer by strengthening the mechanism by which human immunity recognizes and fights against tumor cells. However, immunotherapy has limitations in its effectiveness and is a structure that strengthens immune-related mechanisms; therefore, it often causes serious side effects, leading to skepticism about tumor immunotherapy among oncologists [3]. However, since 2010, surprising results of tumor immunotherapy, especially monoclonal antibodies related to ICIs, have been reported [4]. Thus, monoclonal antibodies related to ICIs are emerging as a new alternative to metastatic cancer treatment.
- Tumor immunotherapy includes not only allogeneic bone marrow transplantation, which has been used for a long time, but it covers various treatment modalities, including tumor vaccines, cytokines, monoclonal antibodies, adoptive cell therapy, and cell therapy. In this article, we review programmed death-1 (PD-1) and programmed death ligand-1 (PD-L1) inhibitors, which are ICIs that are widely applied in clinical practice.
Introduction
- To explain the mechanism of PD-1 and PD-L1, we first discuss the immune cells in the body, especially the T cell lymphocyte immune reaction mechanism. Controlling T cell lymphocyte immune reaction is both complex and precise. For activating T lymphocytes, in advance, the action started with antigen recognition of T cells by binding antigen-bound antigen-presenting cells (APCs) at the T-cell antigen receptor [5]. To fully activate T cells, a stimulatory signal, in which CD80, CD40 expressed on APC surfaces bind the ligand on the T lymphocytes’ surface including CD28, CD40 ligand, was needed. Simultaneously, the inhibitory signal is activated to inactivate the activated T-lymphocyte cells after some time to protect against cell damage resulting from excessive immune reactions [6]. The most recognized inhibitory signals are cytotoxic T-lymphocyte antigen (CTLA)-4, PD-1 expressed on T lymphocytes [7]. In particular, PD-1 is known to regulate the function of T lymphocytes in peripheral tissues by binding ligands of PD-L1 or PD-L2 and PD-1 of T lymphocytes [8]. The immune system of the human body plays a role in the recognition and eradication of mutated tumor cells, but it can also promote tumor growth by selecting tumor cells that can evade immune surveillance [9]. Thus, tumor cells acquire the ability to not eradicate tumor cells by antigen recognition of the immune system.
- Moreover, tumor cells that escape the immune system can induce an immunosuppressive status by producing cytokines and growth factors, including vascular endothelial growth factor (VEGF), recruiting T cells, and myeloid-derived suppressor cells [5,9,10]. They can also overexpress the inhibitory ligands on their surfaces to escape the immune system and effectively eradicate tumor cells [11,12]. The inhibitory ligands expressed on tumor cells include PD-L1, as mentioned above. PD-L1 is expressed on the tumor cell surface, while PD-1 is expressed on activated B or T lymphocytes [13]. Thus, binding PD-1 ligands on tumor cells at the PD-1 receptor on lymphocytes prevents the activation of immune cells and maintains the progression without eradication by the immune system, preventing the identification of non-self antigens [14].
- PD-L1 is frequently found in various human cancers, including melanoma, lung cancer, renal cell cancer (RCC), head and neck cancer, bladder cancer, ovarian cancer, and gastrointestinal cancer [15]. PD-1 inhibitors or PD-L1 inhibitors prevent the binding of inhibitory signals and sustain the function of eradicating tumor cells by maintaining the activation of T lymphocytes [16]. As a result of studies based on human immune mechanisms on tumor cells, in 2010, a PD-1 inhibitor, pembrolizumab, was developed for melanoma treatment for the first time [2], and the other successful results of various cancers in pembrolizumab and nivolumab led to a new chapter in cancer treatment in current days.
Mechanism of PD-1 and PD-L1
- Currently, most multinational pharmaceutical companies have developed PD-1 or PD-L1 inhibitors for the treatment of various tumors. Here, we describe pembrolizumab, nivolumab, and atezolizumab, which are widely used in Korea.
- 1. Nivolumab
- Nivolumab is the first PD-1 inhibitor to be developed to treat refractory metastatic cancers. In December 2014, nivolumab was approved for metastatic or inoperable melanoma by the U.S. Food and Drug Administration (FDA), demonstrating improvement in progression-free survival (PFS) and overall survival (OS) compared to dacarbazine (PFS, 5.1 months vs. 2.2 months; OS, not reached vs. 10.8 months) [17]. Subsequently, showing PFS and OS improvement in the nivolumab group compared to chemotherapy (docetaxel) in metastatic non-small cell lung cancer (NSCLC) second-line treatment [18], nivolumab is gradually expanding and being applied to other cancer types. Currently, its indications are increasing, including malignant melanoma, NSCLC, urothelial cancer, head and neck cancer, hepatocellular cell carcinoma, kidney cancer, Hodgkin’s lymphoma, stomach cancer, and colorectal cancer [19-26] (Table 1).
- 2. Pembrolizumab
- Earlier, the pharmaceutical company developed the CTLA-4 inhibitor, ipilimumab, and tested positive results in melanoma [4]. Subsequently, the PD-1 inhibitor pembrolizumab was used in a phase II study in refractory melanoma patients despite using ipilimumab. The study reported statistically significant results that OS in the pembrolizumab group (2 mg/kg) was improved compared to chemotherapy (13.4 months vs. 11 months) and was approved by FDA [27].
- In Korea, pembrolizumab was approved for first-line treatment of inoperable or metastatic melanoma, metastatic NSCLC with first-line treatment, and second-line treatment of NSCLC with PD-L1 expression rate above 50% and absence of epidermal growth factor receptor mutation or ALK rearrangement, which progressed to first-line chemotherapy [28,29]. Its indications are expanding to include Hodgkin’s lymphoma, urothelial cancer, head and neck cancer, and breast cancer [30-34] (Table 2).
- 3. Atezolizumab
- Atezolizumab is a fully-humanized monoclonal antibody against the protein PD-L1, while nivolumab and pembrolizumab are PD-1 inhibitors. Atezolizumab was first approved in advanced urothelial cancer where tumors have progressed after platinum-based chemotherapy by the FDA, showing an improvement in the 12-month OS rate (41%) in a phase 2 study compared to a landmark 12-month OS rate of 20% from an analysis of 10 phase 2 trials who received second-line chemotherapy for advanced urothelial cancer [35] and showed efficacy in platinum-ineligible patients with metastatic urothelial cancer (OS, 15.9 months; PFS, 2.7 months) [36]. Also, atezolizumab was approved by the FDA in palliative second-line treatment of NSCLC showing improvement of OS compared to conventional chemotherapy with docetaxel (OS, 13.8 months vs. 9.5 months; hazard ratio [HR], 0.73; p=0.0003) and metastatic NSCLC which has high expression of PD-L1 first-line treatment by proving the efficacy compared to chemotherapy (OS, 20 months vs. 13.1 months; HR, 0.59; p=0.01) [37,38] (Table 3).
PD-1 and PD-L1 inhibitors on market: study results and indication
- Although promising results for inhibitors of PD-1 and PD-L1 have been reported, the low response rate of immunotherapy cancer treatment has been reported to be 15% to 20% [39,40]. With the development of immunotherapy leading to PD-1 and PD-L1 inhibitors, efforts to understand tumor immunology last for a better response to immunotherapy. Inflamed tumors that highly infiltrate immune cells and proinflammatory cytokines are known to respond well to immunotherapy. In addition, other immunotherapies, such as CTLA-4 inhibitors, have a better response correlated with posttreatment increases in tumor-infiltrating lymphocytes [41,42]. In other words, an inflamed tumor can have a better response, and immune modulation-induced treatments of weak immunogenic tumors can have similar results, indicating the possibility of therapeutic intervention in immunotherapy. The biomarkers of ICI response have been validated in several studies. Several factors have received much attention, including PD-L1 expression, mutational burden intensity, and deficiencies in antigen presentation [41,43]. PD-L1 expression in tumors was assessed by immunohistochemistry (IHC) staining of PD-L1 positive tumor cells, immune cells, or both cells. High expression PD-L1 in tumors including melanoma, NSLCL, RCC, prostate cancer, and colorectal cancer showed an objective response to nivolumab using a 5% PD-L1 positivity threshold. However, there is a hurdle that multiple variables of PD-L1 IHC staining caused by transient, intrapatient, and intratumoral heterogeneity and poor uniform test of PD-L1 expression result in poor reliability. In addition, as mentioned above regarding the mechanism of PD-1 inhibitors for cancers, neoantigens produced by somatic mutation of tumor cells as primary drivers of anticancer adaptive immune response have been identified in preclinical data. The long-term clinical benefit of high mutational or neoantigen burden with a mutational load of more than 100 nonsynonymous somatic mutations in cancers has been reported in several studies [44,45].
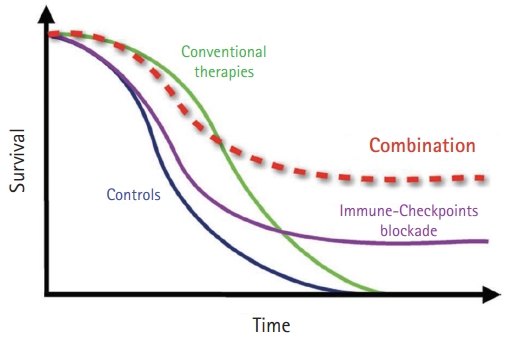
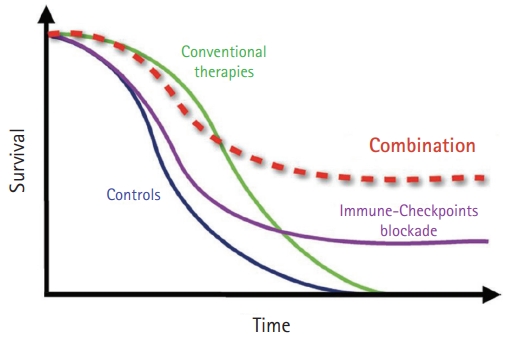
- Some modulators act directly on tumors to increase their immunogenicity, including chemotherapy, radiotherapy, and metabolic modifiers. While conventional chemotherapy has a rapid response initially and has been resistant to tumors in a short time, PD-1 or PD-L1 inhibitors have a lower response rate than conventional chemotherapy and sustained the response in the responding group for long periods. To increase the response rate to immunotherapy, combination treatment with immunotherapy was thought to amplify the antitumor immune response (Fig. 1) [46]. In addition, destroying cancer cells by cytotoxic agents release tumor-associated antigens that can stimulate immune responses to infiltrate the immune cells around tumors so that they change to an inflamed tumor [47]. To make an inflamed tumor is essential to increasing the response to PD1 or PD-L1 inhibitors.
- 1. Combination of chemotherapy
- Recently, many studies have been conducted on combination treatment with immunotherapy, including immunotherapy, chemotherapy, targeted therapy, and radiotherapy. In particular, good candidates with combination treatment are included in chemotherapy or targeted therapy, because conventional therapy could help achieve rapid tumor regression, and immune checkpoint blockade could then help sustain the tumor response, inducing a long-lasting immune-mediated reaction [46]. The study of combination treatment with ICI and chemotherapy started with metastatic NSCLC. In the first-line treatment of metastatic NSLCL, PFS and OS improvement showed the pembrolizumab group with pemetrexed and carboplatin compared to conventional chemotherapy regardless of PD-L1 status (HR, 0.49; p<0.001). In 2018, this combination therapy was first approved by the FDA and is now routinely used as a first-line treatment in metastatic NSCLC [48,49]. Accordingly, the combination of nab-paclitaxel and atezolizumab was recently approved for first-line treatment of triple negative metastatic breast cancer, showing improved OS compared to the nab-paclitaxel only group in PD-L1 positive patients (OS, 25 months vs. 15.5 months) [50]. The PFS of the combination of nab-paclitaxel and atezolizumab group showed the benefit in all populations (7.5 months vs. 5.0 months; HR, 0.62; p<0.001) and failed to show the benefit of OS in all populations. However, this study proved that adding chemotherapy in immunotherapy improved survival benefit in the PD-L1 positive group compared to immunotherapy alone. The great success of combination therapy in NSCLC and breast cancer has led to more studies on combination treatment with immunotherapy. In metastatic small cell lung cancer (SCLC), combination treatment with atezolizumab, etoposide, and carboplatin revealed better survival benefit compared to conventional chemotherapy with etoposide and carboplatin in IMpower 133 study (OS, 12.3 months vs. 10.3 months; HR, 0.70; p=0.007) [51]. Similarly, durvalumab with etoposide and platinum improved the OS compared to conventional chemotherapy in metastatic SCLC first-line treatment in the CASPIAN study (OS months, 13.3 vs. 10.3 months; HR, 0.73; p=0.0047) [52]. In metastatic urothelial cancer, positive results were sustained when reporting the OS improvement of atezolizumab with platinum-containing chemotherapy compared to placebo and platinum-containing chemotherapy in palliative first-line treatment of metastatic urothelial cancer of IMvigor 130 study (PFS: 8.2 months vs. 6.3 months; HR, 0.82; p=0·007; OS: 16 months vs. 13.4 months; HR, 0.83; p=0.027) [53]. However, the OS results were not significant. In addition, the study results of pembrolizumab and platinum-containing chemotherapy vs. pembrolizumab-only vs. chemotherapy only were reported in palliative first-line treatment of metastatic urothelial cancer. HR (95% confidence interval [CI]) for pembrolizumab and chemotherapy vs. chemotherapy was only 0.78 (0.65–0.93, p=0.0033) for PFS and 0.86 (0.72–1.02, p=0.0407) for OS but, unfortunately, these results were not significant for OS and PFS [54]. PD-1 or PDL-1 inhibitors in urothelial cancer after palliative treatment seemed to have functional limitations. Recently, in the case of stomach cancer, good results were reported in the CheckMate 649 study that showed capecitabine, oxaliplatin, and nivolumab improved OS and PFS compared with conventional chemotherapy in first-line metastatic stomach cancer treatment (OS: HR, 0.80; p=0.0002; PFS: HR, 0.68; p<0.0001) [55].
- 2. Combination of targeted therapy
- In targeted immunotherapy agents, good results have been reported, especially in kidney cancer. Improvement of OS and PFS with the VEGF inhibitor axitinib and pembrolizumab compared to sunitinib, a first-line metastatic RCC (mRCC) conventional treatment (OS: HR, 0.68; p=0.0003; PFS: HR, 0.71; p<0.001) [56,57]. Similarly, lenvatinib plus pembrolizumab compared to sunitinib in first-line mRCC treatment showed a significant improvement in OS (HR, 0.66; p=0.005) [58]. Another study of nivolumab and cabozantinib vs. sunitinib in palliative first-line mRCC showed good results in palliative first-line mRCC treatment. HR (95% CI) for nivolumab and cabozantinib vs. sunitinib was 0.51 (0.41–0.64, p<0.001) for OS and 0.60 (0.40–0.89, p=0.001) [59]. In endometrial cancer, one of the gynecologic malignancies, targeted agents with immunotherapy have been reported to improve survival compared to conventional chemotherapy. Lenvatinib plus pembrolizumab improved OS compared to doxorubicin and paclitaxel treatment in second-line metastatic endometrial cancer in the Keynote 775 study (HR, 0.56; p<0.0001) [60]. The status of deficient mismatch repair (dMMR) in tumors was investigated in this study, and the study showed that the lenvatinib plus pembrolizumab group improved survival regardless of dMMR. The results of the combination with immunotherapy trials are listed in Table 4. Not only VEGF inhibitors but also other targeted agents including poly ADP-ribose polymerase (PARP) inhibitor and CDK4/6 inhibitor with ICIs have benefited from synergistic PD-1/PD-L1 inhibitors in preclinical and early clinical data [61]. In particular, phase II studies of PARP inhibitors with PD-1/PDL-1 inhibitor combination treatment in ovarian and breast cancer have shown positive results, and the results of ongoing randomized control trials will be expected [62-64].
New direction of PD-1 and PD-L1 inhibitors
- Since 2015, immunotherapy, especially ICI, has become a mainstream cancer treatment. The studies of ICIs showed good responses of various tumor types when applied to many types of cancer. Immunotherapy has a durable response and low toxicity, but immunotherapy alone has a response of 15% to 20% in the cancer patient treatment group. Thus, the challenge of improving the response to immunotherapy was sustained, and we found that the microenvironment in immune cells is very important to act appropriately in cancer. A combination of chemotherapy or targeted therapy with immunotherapy was started on cancer treatment to make an inflamed tumor help increase the response to immunotherapy. Recently, studies on combination therapy reported improved OS and PFS, even though they did not respond to immunotherapy. Many trials of combination therapy are ongoing and will be expected to continue the benefits of survival (Table 5). Thus, combining chemotherapy or targeted agents with immunotherapy is one of the leading pathways for cancer treatment.
Conclusion
-
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Notes
| Phase | Population | Therapy (No. of patients) | ORR (%) | OS (mo) | PFS (mo) | HR for OS (p-value) |
|---|---|---|---|---|---|---|
| III [17] | Metastatic melanoma (BRAF-) / first line | Nivo (210) | 50 | NR | 5.1 | 0.42 (<0.001) |
| DTIC (208) | 13.9 | 10.8 | 2.2 | |||
| III [18] | Metastatic NSCLC (squamous) / second line | Nivo (135) | 20 | 9.2 | 3.5 | 0.59 (<0.001) |
| Docetaxel (137) | 9 | 6.0 | 2.8 | |||
| III [19] | Recurrent H&N cancer / second line | Nivo (240) | 7.5 | 2 | 0.70 (0.01) | |
| Standard chemotherapy (investigator choice) (121) | 5.1 | 2.3 | ||||
| II (single arm) [20] | Recurrent classical Hodgkin lymphoma | Nivo (80) | 66 | |||
| III [21] | Refractory to at least 2nd line chemotherapy advanced gastric or GEJ cancer | Nivo (330) | 11.2 | 5.26 | 1.61 | 0.63 (<0.0001) |
| Placebo (163) | 0 | 4.14 | 1.45 | |||
| II (single arm) [22] | MSI-Ha) metastatic colorectal cancer / pretreated refactory | Nivo (53) | 28 | |||
| II (single arm) [23] | Unresectable or metastatic urothelial cancer / second line | Nivo (270) | 19.6 | 5.95 (PD-L1 <1%) | 2 | |
| 11.3 (PD-L1 ≥1%) | ||||||
| I/II [24] | Sorafenib failure HCC | Nivo (154) | 14.3 | |||
| III [25] | Metastatic RCC / second line | Nivo (410) | 25 | 25 | 4.6 | 0.73 (0.002) |
| Everolimus (411) | 5 | 19.6 | 4.4 | |||
| III [26] | Metastatic NSCLC (nonsquamous) / second line | Nivo (292) | 19 | 12.2 | 2.3 | 0.73 (0.002) |
| Docetaxel (290) | 12 | 9.4 | 4.2 |
ORR, overall response rate; OS, overall survival; PFS, progression-free survival; HR, hazard ratio; BRAF, B-type Raf kinase; Nivo, nivolumab; DTIC,dacarbazine; SLCL, non-small cell lung cancer; H&N, head and neck; GEJ, gastroesophageal junction; MSI-H, microsatellite instability-high; PD-L1, programmed death ligand-1; HCC, hepatocellular carcinoma; RCC, renal cell cancer.
a) MSI-H is defined if two or more markers are positive using polymerase chain reaction on tumor tissue.
| Phase | Population | Therapy (No. of patients) | ORR (%) | OS (mo) | PFS (mo) | HR for OS (p-value) |
|---|---|---|---|---|---|---|
| II [27] | Ipilimumab failed unresectable melanoma | Pem 2 mg/kg (180) | 21 | 13.4 | 5.4 | 0.57 (0.001) |
| Pem 10 mg/kg (181) | 25 | 14.7 | 5.8 | 0.50 (0.001) | ||
| Chemotherapy (179) | 4 | 11 | 3.6 | |||
| III [28] | Metastatic NSCLC / second line | Pem 2 mg/kg (345) | 30 | 10.4 | 5.0 | 0.71 (0.0008) |
| Pem 10 mg/kg (346) | 29 | 12.7 | 5.2 | 0.61 (0.0001) | ||
| Docetaxel (343) | 8 | 8.5 | 4.1 | |||
| III [29] | Metastatic NSCLC / first line (PD-L1 ≥50%) | Pem (154) | 44.8 | NR | 10.3 | 0.60 (0.005) |
| Chemotherapy (151) | 27.8 | 14.5 | 6.0 | |||
| II (single arm) [30] | Recurrent H&N cancer / second line | Pem (210) | 69 | NR | ||
| III [31] | Metastatic urothelial cancer / second line | Pem (270) | 21.1 | 8.0 | 2.1 | 0.73 (0.002) |
| Chemotherapy (272) | 11.4 | 5.2 | 3.3 | |||
| III [33] | Metastatic triple negative breast cancer / first line | Pem+chemotherapy (566) | 9.7 | 0.65 (0.0012)a) | ||
| Chemotherapy (281) | 5.6 | |||||
| III [34] | Stage II or III triple negative breast cancer neoadjuvant | Pem+Pac/Car (401) | 64.8 (pCR)b) | –0.001 | ||
| Pac/Car (201) | 51.2 (pCR) |
ORR, overall response rate; OS, overall survival; PFS, progression-free survival; HR, hazard ratio; NSLCL, non-small cell lung cancer; PD-L1, programmed death ligand-1; NR, not reached; H&N, head and neck; Pac, paclitaxel; Car, carboplatin; pCR, pathologic complete remission.
a) Pem+chemotherapy resulted in a significant improvement of PFS compared to chemotherapy alone in patients with combined positive score (PD-L1 status) of 10 or more.
b) pCR is pathological stage ypT0/Tis ypN0.
| Phase | Population | Therapy (No. of patients) | ORR (%) | OS (mo) | PFS (mo) | HR for OS (p-value) |
|---|---|---|---|---|---|---|
| II (single arm) [35] | Platinum failed advanced urothelial cancer / second line | Ate 1,200 mg (315) | 15 | 11.7 | 2.1 | |
| II (single arm) [36] | Platinum ineligible advanced urothelial cancer / first line | Ate 1,200 mg (119) | 23 | 15.9 | ||
| III [37] | Metastatic NSCLC / first line (high expression of PD-L1)a) | Ate 1,200 mg (277) | 20.2 | 8.1 | 0.59 (0.01) | |
| Chemotherapy (277) | 13.1 | 5.0 | ||||
| II [38] | Metastatic NSCLC / second line | Ate 1,200 mg (425) | 13.8 | 2.8 | 0.73 (0.0003) | |
| Docetaxel (425) | 9.5 | 4.0 |
ORR, overall response rate; OS, overall survival; PFS, progression-free survival; HR, hazard ratio; NSLCL, non-small cell lung cancer; PD-L1, programmed death ligand-1.
a) High expression of PD-L1 defined as ≥1% PD-L1 expression on tumor cells and any level of PD-L1 expression on tumor-infiltrating immune cells, <1% PD-L1 expression on tumor cells, and ≥1% PD-L1 expression on tumor-infiltrating immune cells by SP142 assay.
| Phase | Population | Therapy (No. of patients) | OS (mo) | HR (p-value) |
|---|---|---|---|---|
| III [48] | Metastatic NSCLC / first line (nonsquamous) (Keynote 189) | Pem+pemetrexed/Car (410) | NR | 0.49 (0.001) |
| Pemetrexed/Car (206) | 11.3 | |||
| III [49] | Metastatic NSCLC / first line (squamous) (Keynote 407) | Pem+Pac/Car (278) | 15.9 | 0.64 (0.001) |
| Pac/Car (281) | 11.3 | |||
| III [50] | Metastatic triple negative breast cancer / first line (IMpssion130) | Ate+nab-Pac (185) (PD-L1 positive) | 25 | 0.62 |
| Nab-Pac (184) (PD-L1 positive) | 15.5 | |||
| III [51] | Metastatic SCLC / first line (IMpower133) | Ate+EP (201) | 12.3 | 0.7 (0.007) |
| EP (202) | 10.3 | |||
| III [52] | Metastatic SCLC / first line (CASPIAN) | Durvalumab+EP (268) | 13.3 | 0.73 (0.0047) |
| EP (269) | 10.3 | |||
| III [53] | Metastatic urothelial cancer / first line (IMvigor 130) | Ate+Gem/Platinum (451) | 16 | 0.83 (vs. chemotherapy) |
| Ate (362) | (0.0027) | |||
| Gem/Platinum (400) | 13.4 | |||
| III [54] | Metastatic urothelial cancer / First line (Keynote 361) | Pem+Gem/Platinum (351) | 17 | 0.86 (vs. chemotherapy) |
| Pem (307) | 15.6 | (0.0407) | ||
| Gem/Platinum (352) | 14.3 | |||
| III [55] | Metastatic gastric cancer (CheckMate 649) | Nivo+Xelox or Folfox (789) | 13.8 | 0.80 (0.0002) |
| Chemotherapy (792) | 11.6 | |||
| III [57] | Metastatic renal cell cancer (Keynote 426) | Pem+axitinib (432) | NR | 0.68 (0.0003) |
| Sunitinib (429) | 35.7 | |||
| III [58] | Metastatic renal cell cancer / first line (CLEAR) | Pem+lenvatinib(355) | NR | 0.66 (0.001) |
| Lenvatinib+everolimus (357) | NR | |||
| Sunitinib (357) | NR | |||
| III [60] | Metastatic endometrial cancer / second line (Keynote 775) | Pem+lenvatinib (411) | 18.3 | 0.62 (0.0001) |
| Chemotherapy (416) | 11.4 |
- 1. Hong S, Won YJ, Lee JJ, Jung KW, Kong HJ, Im JS, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2018. Cancer Res Treat 2021;53:301–15.ArticlePubMedPMC
- 2. Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol 2019;30:582–8.ArticlePubMedPMC
- 3. Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell 2018;175:313–26.ArticlePubMedPMC
- 4. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23.ArticlePubMedPMC
- 5. Vesely MD, Schreiber RD. Cancer immunoediting: antigens, mechanisms, and implications to cancer immunotherapy. Ann N Y Acad Sci 2013;1284:1–5.ArticlePMC
- 6. Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol 2006;90:297–339.ArticlePubMedPMC
- 7. Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008;322:271–5.ArticlePubMed
- 8. Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793–800.ArticlePubMed
- 9. Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol 2011;29:235–71.ArticlePubMed
- 10. Radoja S, Rao TD, Hillman D, Frey AB. Mice bearing late-stage tumors have normal functional systemic T cell responses in vitro and in vivo. J Immunol 2000;164:2619–28.ArticlePubMed
- 11. Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 2009;206:3015–29.ArticlePubMedPMC
- 12. Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol 2008;8:467–77.ArticlePubMed
- 13. Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2001;2:261–8.ArticlePubMed
- 14. Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 1999;5:1365–9.ArticlePubMed
- 15. Wang X, Teng F, Kong L, Yu J. PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther 2016;9:5023–39.ArticlePubMedPMC
- 16. Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A 2002;99:12293–7.ArticlePubMedPMC
- 17. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–30.ArticlePubMed
- 18. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123–35.ArticlePubMedPMC
- 19. Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–67.ArticlePubMedPMC
- 20. Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015;372:311–9.ArticlePubMed
- 21. Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:2461–71.ArticlePubMed
- 22. Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182–91.ArticlePubMedPMC
- 23. Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017;18:312–22.ArticlePubMed
- 24. El-Khoueiry AB, Melero I, Crocenzi TS, Welling TH, Yau TC, Yeo W, et al. Phase I/II safety and antitumor activity of nivolumab in patients with advanced hepatocellular carcinoma (HCC): CA209-040. J Clin Oncol 2015;33(Suppl):LBA101.Article
- 25. Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1803–13.ArticlePubMedPMC
- 26. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627–39.ArticlePubMedPMC
- 27. Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015;16:908–18.ArticlePubMedPMC
- 28. Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540–50.ArticlePubMed
- 29. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823–33.ArticlePubMed
- 30. Chen R, Zinzani PL, Fanale MA, Armand P, Johnson NA, Brice P, et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol 2017;35:2125–32.ArticlePubMedPMC
- 31. Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–26.ArticlePubMedPMC
- 32. Mehra R, Seiwert TY, Gupta S, Weiss J, Gluck I, Eder JP, et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long-term follow-up in KEYNOTE-012. Br J Cancer 2018;119:153–9.ArticlePubMedPMC
- 33. Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020;396:1817–28.PubMed
- 34. Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med 2020;382:810–21.ArticlePubMed
- 35. Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909–20.ArticlePubMedPMC
- 36. Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389:67–76.ArticlePubMed
- 37. Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med 2020;383:1328–39.ArticlePubMed
- 38. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837–46.ArticlePubMed
- 39. Yun S, Vincelette ND, Green MR, Wahner Hendrickson AE, Abraham I. Targeting immune checkpoints in unresectable metastatic cutaneous melanoma: a systematic review and meta-analysis of anti-CTLA-4 and anti-PD-1 agents trials. Cancer Med 2016;5:1481–91.ArticlePubMedPMC
- 40. Lu J, Lee-Gabel L, Nadeau MC, Ferencz TM, Soefje SA. Clinical evaluation of compounds targeting PD-1/PD-L1 pathway for cancer immunotherapy. J Oncol Pharm Pract 2015;21:451–67.ArticlePubMed
- 41. Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 2016;17:e542–51.ArticlePubMedPMC
- 42. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568–71.ArticlePubMedPMC
- 43. Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 2019;51:202–6.ArticlePubMedPMC
- 44. Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014;371:2189–99.ArticlePubMedPMC
- 45. Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 2015;350:207–11.ArticlePubMedPMC
- 46. Champiat S, Ileana E, Giaccone G, Besse B, Mountzios G, Eggermont A, et al. Incorporating immune-checkpoint inhibitors into systemic therapy of NSCLC. J Thorac Oncol 2014;9:144–53.ArticlePubMed
- 47. Mantovani A, Romero P, Palucka AK, Marincola FM. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet 2008;371:771–83.ArticlePubMed
- 48. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018;378:2078–92.ArticlePubMed
- 49. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 2018;379:2040–51.ArticlePubMed
- 50. Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 2018;379:2108–21.ArticlePubMed
- 51. Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 2018;379:2220–9.ArticlePubMed
- 52. Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019;394:1929–39.PubMed
- 53. Galsky MD, Arija JÁA, Bamias A, Davis ID, De Santis M, Kikuchi E, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2020;395:1547–57.ArticlePubMed
- 54. Powles T, Csőszi T, Özgüroğlu M, Matsubara N, Géczi L, Cheng SY, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:931–45.PubMed
- 55. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021;398:27–40.ArticlePubMedPMC
- 56. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019;380:1116–27.ArticlePubMed
- 57. Powles T, Plimack ER, Soulières D, Waddell T, Stus V, Gafanov R, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol 2020;21:1563–73.ArticlePubMed
- 58. Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med 2021;384:1289–300.ArticlePubMed
- 59. Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2021;384:829–41.ArticlePubMedPMC
- 60. Makker V, Herraez AC, Aghajanian C, Fujiwara K, Pignata S, Penson RT, et al. A phase 3 trial evaluating efficacy and safety of lenvatinib in combination with pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol 2019;37(15 Suppl):TPS5607.Article
- 61. Dickler MN, Tolaney SM, Rugo HS, Cortés J, Diéras V, Patt D, et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR+/HER2- metastatic breast cancer. Clin Cancer Res 2017;23:5218–24.ArticlePubMedPMC
- 62. Diaz LA, Le D, Maio M, Ascierto PA, Geva R, Motola-Kuba D, et al. Pembrolizumab in microsatellite instability high cancers: updated analysis of the phase II KEYNOTE-164 and KEYNOTE-158 studies. Ann Oncol 2019;30(Suppl 5):v475.Article
- 63. Konstantinopoulos PA, Waggoner S, Vidal GA, Mita M, Moroney JW, Holloway R, et al. Single-arm phases 1 and 2 trial of niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma. JAMA Oncol 2019;5:1141–9.ArticlePubMedPMC
- 64. Vinayak S, Tolaney SM, Schwartzberg L, Mita M, McCann G, Tan AR, et al. Open-label clinical trial of niraparib combined with pembrolizumab for treatment of advanced or metastatic triple-negative breast cancer. JAMA Oncol 2019;5:1132–40.ArticlePubMedPMC
References
Figure & Data
References
Citations

- Immune checkpoint inhibitors associated cardiovascular immune-related adverse events
Wonyoung Jo, Taejoon Won, Abdel Daoud, Daniela Čiháková
Frontiers in Immunology.2024;[Epub] CrossRef - Therapeutic Targeting of Thioredoxin Reductase 1 Causes Ferroptosis while Potentiating Anti-PD-1 Efficacy in Head and Neck Cancer
Ming-Shou Hsieh, Hang Huong Ling, Syahru Agung Setiawan, Mardiah Suci Hardianti, Iat-Hang Fong, Chi-Tai Yeh, Jia-Hong Chen
Chemico-Biological Interactions.2024; : 111004. CrossRef

 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine PubReader
PubReader ePub Link
ePub Link Cite
Cite