PubMed Central, CAS, DOAJ, KCI

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Volume 38(3); 2021 > Article
-
Case report
Successful treatment with vedolizumab in an adolescent with Crohn disease who had developed active pulmonary tuberculosis while receiving infliximab -
Sujin Choi1,2
 , Bong Seok Choi1
, Bong Seok Choi1 , Byung-Ho Choe1,2
, Byung-Ho Choe1,2 , Ben Kang1,2
, Ben Kang1,2
-
Yeungnam University Journal of Medicine 2021;38(3):251-257.
DOI: https://doi.org/10.12701/yujm.2020.00878
Published online: February 19, 2021
1Department of Pediatrics, School of Medicine, Kyungpook National University, Daegu, Korea
2Crohn’s and Colitis Association in Daegu-Gyeongbuk (CCAiD), Daegu, Korea
- Corresponding author: Ben Kang, MD Department of Pediatrics, School of Medicine, Kyungpook National University, 680 Gukchaebosang-ro, Jung-gu, Daegu 41944, Korea Tel: +82-53-200-2749 Fax: +82-53-200-2029 E-mail: benkang@knu.ac.kr
Copyright © 2021 Yeungnam University College of Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 5,057 Views
- 131 Download
- 2 Crossref
Abstract
- Vedolizumab (VDZ) has been approved for the treatment of inflammatory bowel diseases (IBDs) in patients aged ≥18 years. We report a case of a pediatric patient with Crohn disease (CD) who was successfully treated with VDZ. A 16-year-old female developed severe active pulmonary tuberculosis (TB) during treatment with infliximab (IFX). IFX was stopped, and TB treatment was started. After a 6-month regimen of standard TB medication, her pulmonary TB was cured; however, gastrointestinal symptoms developed. Due to the concern of the patient and parents regarding TB reactivation on restarting treatment with IFX, VDZ was started off-label. After the second dose of VDZ, the patient was in clinical remission and her remission was continuously sustained. Ileocolonoscopy at 1-year after VDZ initiation revealed endoscopic healing. Therapeutic drug monitoring conducted during VDZ treatment showed negative antibodies to VDZ. No serious adverse events occurred during the VDZ treatment. This is the first case report in Korea demonstrating the safe and effective use of VDZ treatment in a pediatric CD patient. In cases that require recommencement of treatment with biologics after recovery of active pulmonary TB caused by anti-tumor necrosis factor agents, VDZ may be a good option even in pediatric IBD.
- Crohn disease (CD) is an inflammatory bowel disease (IBD) characterized by ulcers and inflammation that develop anywhere throughout the gastrointestinal (GI) tract [1]. Approximately 25% of patients with CD are diagnosed at <20 years of age, in whom a more aggressive disease course occurs compared with adult-onset disease. Hence, an earlier introduction of immunomodulators and/or anti-tumor necrosis factor (TNF) agents is required [1-3].
- Vedolizumab (VDZ) is a humanized monoclonal antibody acting on α4β7 integrin that is present on the surface of lymphocytes and binds to MadCAM-1 on the intestinal endothelium. VDZ selectively inhibits the transmigration of lymphocytes into the inflamed intestinal tissue [4]. This gut-selective mechanism of action of VDZ is advantageous in terms of safety compared to other biologics. VDZ is currently approved for use only in adults with CD and ulcerative colitis (UC) [5,6]. Anti-TNF agents, such as infliximab (IFX) and adalimumab, are the only biological agents approved for use in pediatric IBD, whereas VDZ can be administered only off-label [7,8]. Herein, we report a rare case of a pediatric CD patient who successfully achieved and maintained remission with VDZ after developing severe active pulmonary tuberculosis (TB) during treatment with IFX.
Introduction
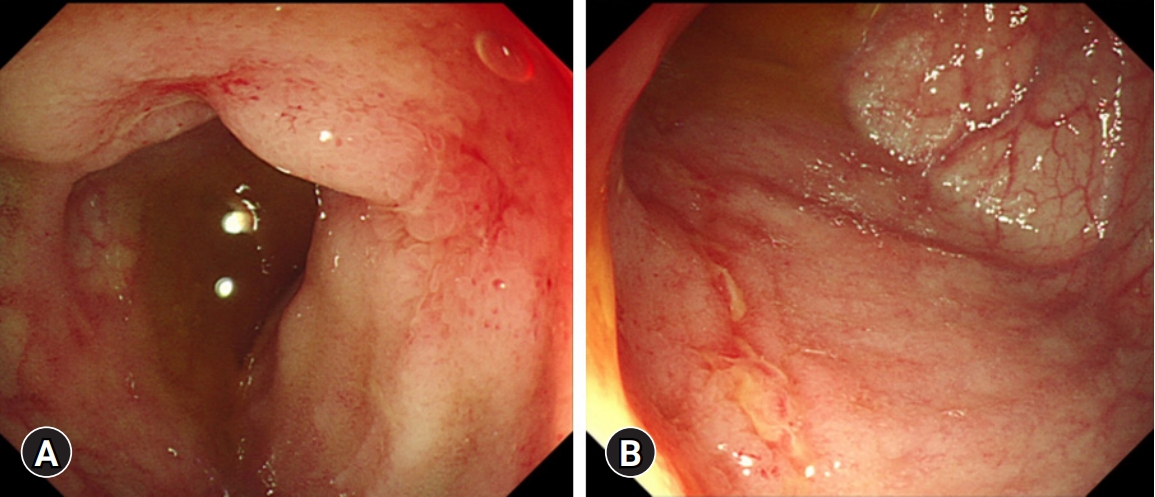
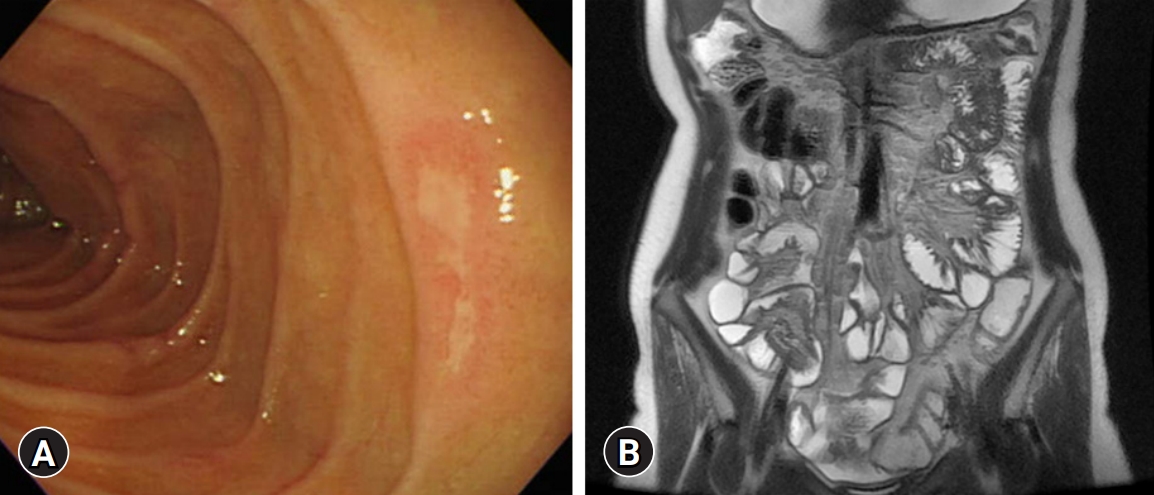
- A 16-year-old female was admitted to Kyungpook National University Children’s Hospital with complaints of abdominal pain, diarrhea, and hematochezia for 2 months. The past medical history of the patient was unremarkable. She had completed Bacillus Calmette–Guérin (BCG) vaccination and had no past or family history of pulmonary TB. Initial laboratory tests showed a white blood cell count of 4,500/μL, hemoglobin level of 6.2 g/dL, platelet count of 532,000/μL, albumin level of 3.0 g/dL, erythrocyte sedimentation rate (ESR) of 110 mm/hr, and C-reactive protein (CRP) level of 8.0 mg/dL. The fecal immunochemical test (FIT) was positive, and fecal calprotectin (FC) was >2,000 mg/kg. No pathogens were detected in the stool culture and stool polymerase chain reaction (PCR). Chest X-ray showed no abnormal findings in the lungs, and the interferon-gamma release assay (IGRA) was negative. Ileocolonoscopy showed multifocal ulcers throughout the terminal ileum and colon (Fig. 1). Cryptitis, crypt abscesses, and non-caseating granulomas were observed throughout the terminal ileum and colon on histology; however, the acid-fast bacillus smear and culture and PCR for TB were negative. Upper GI endoscopy revealed ulcers in the duodenum (Fig. 2A). Magnetic resonance enterography showed multisegmental wall thickening in the ileum (Fig. 2B). The patient was diagnosed with CD with a phenotype of A1b, L3+L4ab, B1, G0 according to the Paris classification. Her Pediatric Crohn’s Disease Activity Index (PCDAI) score was 50, and the Simple Endoscopic Score for Crohn’s Disease (SES-CD) was 22.
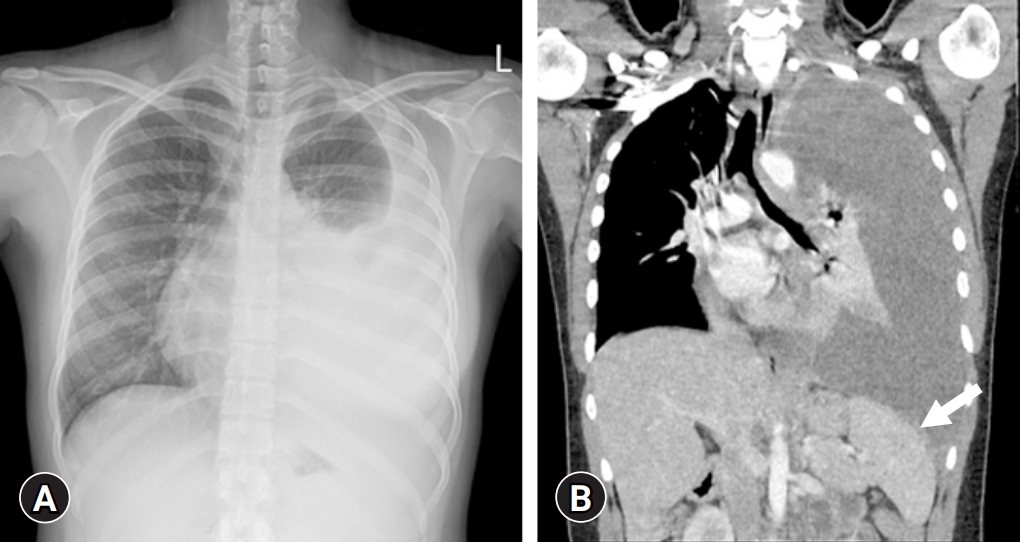
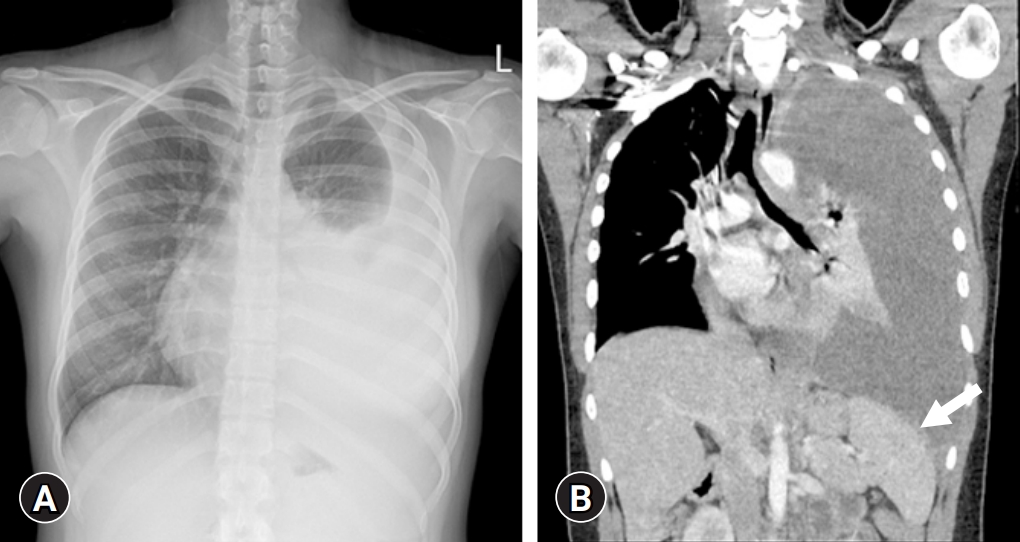
- Treatment began with exclusive enteral nutrition (EEN), mesalazine, and azathioprine, which were effective. One month after treatment, her PCDAI score had decreased to 10. However, 1 month after finishing her 8-week treatment with EEN, the disease relapsed and her PCDAI score elevated to 42.5; hence, prednisolone was started per oral at a dose of 50 mg/day. Despite corticosteroid treatment, symptoms persisted, and IFX was administered. Chest X-ray before the administration of IFX showed no abnormal findings in the lungs, and the IGRA was negative. Symptoms resolved after the second dose of IFX infusion, and her PCDAI score decreased to 7.5. However, during her visit for the third IFX infusion, she complained of fever, cough, and dyspnea on exertion for a week. Radiologic examination of the chest revealed a left pleural effusion with mild pleural thickening and nodularities indicating TB pleurisy on chest X-ray and computed tomography (Fig. 3). Thoracentesis was conducted, and pulmonary TB was confirmed by a positive TB PCR test of the pleural effusion specimen and a positive IGRA test. IFX treatment was discontinued and the standard TB treatment regimen consisting of isoniazid, ethambutol, rifampin, and pyrazinamide for 2 months, followed by isoniazid, ethambutol, and rifampin for 4 months, was started. EEN was restarted and maintained for 8 weeks. Subsequently, mesalazine was administered to treat CD.
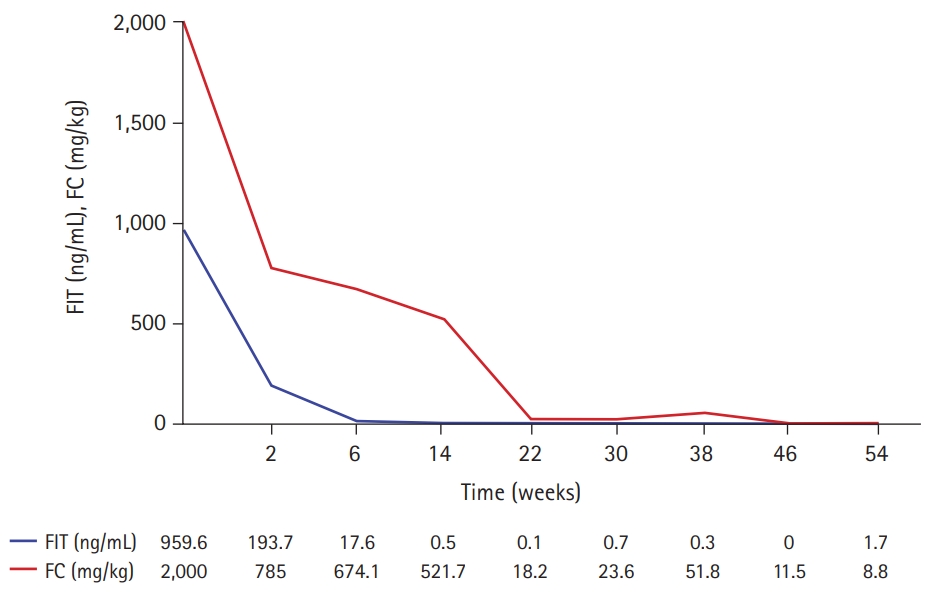
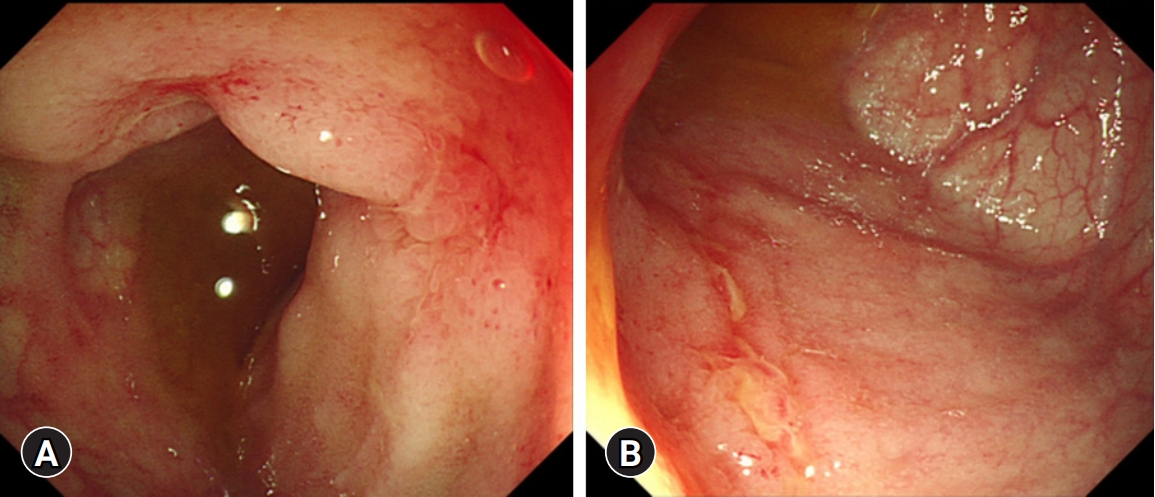
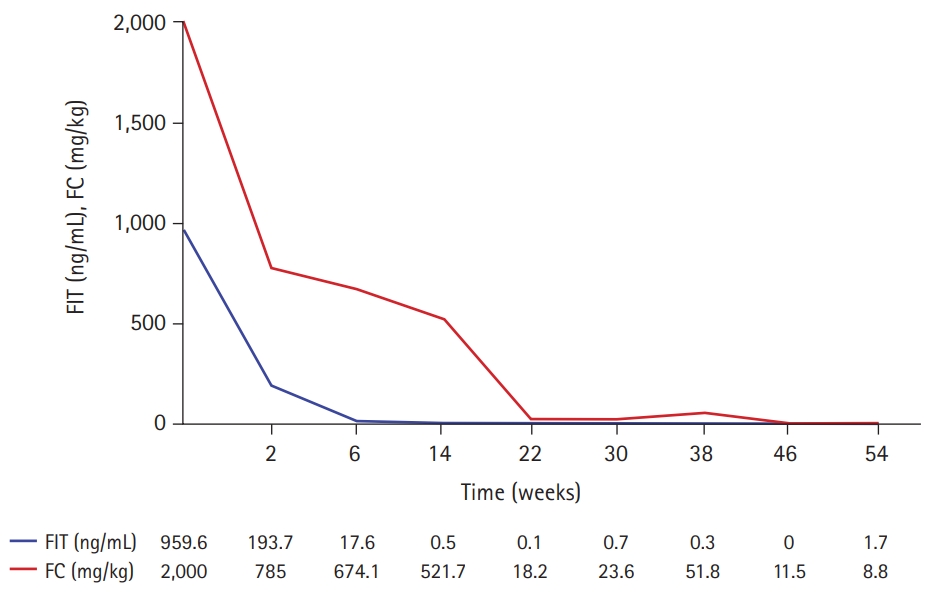
- Her pulmonary TB was completely cured after 6 months of TB treatment. However, symptoms such as hematochezia, diarrhea, and weight loss began by the end of TB treatment. Laboratory tests showed a white blood cell count of 7,010/μL, hemoglobin level of 6.8 g/dL, platelet count of 477,000/μL, albumin level of 3.1 g/dL, ESR of 45 mm/hr, and CRP of 2.2 mg/dL. FIT was positive, and FC was >2,000 mg/kg. No pathogens were detected in the stool culture and stool PCR. Her PCDAI score was 47.5, and the exacerbation of CD was confirmed by ileocolonoscopy (Fig. 4A). Therefore, we planned to restart IFX. However, due to the fear of another severe disease course of active TB when IFX were to be restarted, the patient and parents preferred to start VDZ off-label instead of restarting IFX. Before starting VDZ, the patient’s weight was 57 kg. VDZ was administered according to the regular regimen approved in adults of 300 mg per dose at weeks 0, 2, and 6 for induction and 8-week intervals for maintenance treatment thereafter. The patient showed a fast response to VDZ treatment. At the week 6 visit for her third VDZ infusion, she was in clinical remission, and inflammatory markers were normalized (Table 1, Fig. 5). Ileocolonoscopy at the 1-year follow-up after VDZ treatment revealed endoscopic healing (Fig. 4B). Therapeutic drug monitoring (TDM) using commercialized enzyme-linked immunosorbent assay kits (Immundiagnostik AG, Bensheim, Germany) was conducted during VDZ treatment. An anti-drug antibody level of ≤10 AU/mL was defined as negative, according to the manufacturer’s manual. TDM results during VDZ treatment showed negative antibodies to VDZ (Table 1). The patient is currently in her second year of VDZ treatment and is maintaining clinical and biochemical remission. No serious adverse events, including TB reactivation, have occurred.
Case
- With the introduction of anti-TNF agents in the treatment of CD, there is no doubt that treatment outcomes have improved. However, anti-TNF agents are known to increase the risk of serious infections, including TB [9]. It has been reported that anti-TNF agents are associated with a 2- to 8-fold increased risk of developing active TB [9]. Reactivation of latent TB infections (LTBI) rather than a new infection is considered to be the primary cause of active TB, as most of the active TB cases have been reported to occur within 3 to 4 months after starting anti-TNF treatment [9]. Therefore, screening for LTBI prior to anti-TNF treatment is strongly recommended worldwide, and especially in Asia, where the prevalence of LTBI is higher than that of Western countries [9]. In this case report, the patient had completed TB vaccination, did not have any history or exposure to active TB, and TB screening was negative with both chest X-ray and IGRA at both times of diagnostic evaluation of CD and before the commencement of IFX. However, the patient, unfortunately, developed symptoms of active pulmonary TB 5 weeks after starting IFX treatment, indicating that the active TB was probably due to the reactivation of LTBI, which was missed on TB screening.
- It is well known that when active TB is diagnosed during anti-TNF treatment, the anti-TNF agent should be withheld, and anti-TB therapy should be started [10]. Regarding when to restart anti-TNF treatment, it is considered safe to delay the resumption of anti-TNF therapy until the completion of anti-TB treatment [10]. However, if an early resumption of anti-TNF treatment is required, anti-TNF treatment may be restarted as early as 2 months after anti-TB treatment in patients who did not have an initially severe active TB, demonstrated a favorable response to anti-TB treatment, and when drug susceptibility was proven [10]. In this case report, the patient’s symptoms of CD were fortunately well controlled without IFX until the end of TB medication. According to the treatment guidelines, she could have restarted IFX when CD relapsed at the end of TB medication. However, because she had suffered a severe active TB and due to the fear of another severe disease course of active TB when IFX were to be restarted, the patient and parents preferred to start VDZ instead.
- VDZ is effective for achieving both clinical remission and mucosal healing in adult patients with UC and CD [5,6,11]. Regarding safety, according to the integrated safety data from six trials, VDZ did not increase the risk of serious infections, progressive multifocal leukoencephalopathy, or malignancy [12]. Among 2,830 patients with 4,811 person-years (PYs) exposure to VDZ, TB was reported in only four patients (0.14%) with an estimated incidence of 0.1/100 PYs [12]. Meanwhile, the estimated incidence of TB during treatment with anti-TNF agents has been reported as 1.34/100 PYs and 0.79/100 PYs for IFX and adalimumab, respectively [13].
- Compared with adults, there is limited experience with VDZ therapy in pediatric IBD. In children, VDZ is only available off-label and is used for patients who have already exhausted other treatment options, including anti-TNF agents. According to the ECCO/ESPGHAN (European Crohn’s and Colitis Organisation and the European Society of Pediatric Gastroenterology, Hepatology and Nutrition) guideline, VDZ should be considered in chronically active or steroid-dependent pediatric UC patients as a second-line biologic therapy after anti-TNF failure, even though it has not yet been approved for use in children [7]. In addition, in pediatric CD patients who fail to maintain clinical remission on anti-TNF agents despite dose optimization and immunomodulator use, VDZ can be considered off-label [8]. A multicenter retrospective study showed that VDZ was safe and effective in pediatric IBD patients [14]. In this study, 64 children with IBD who had been previously treated with anti-TNF agents were included. During a median follow-up period of 24 months, corticosteroid-free remission was 37% in UC and 14% in CD at week 14, and 39% in UC and 24% in CD at the last follow-up [14]. Another retrospective study on 52 children with IBD showed clinical remission rates of 76% and 42% for UC and CD, respectively, at week 14 [15]. No serious adverse events were reported in either study [14,15].
- In the GEMINI 3 trial, VDZ was not more effective than placebo in inducing clinical remission at week 6 among patients with CD who had failed previous treatment with anti-TNF agents [16]. The clinical benefits of VDZ in these patients were detectable later at week 10 [16]. This slow induction rate of VDZ was also observed in pediatric patients with CD [14]. Contrary to these findings, the patient in this case report showed a fast response to VDZ. Additionally, according to the clinical decision support tool developed for predicting the probability of response to VDZ in adult CD patients [17], the patient’s score in this case report was 16, which applies to an intermediate response probability. However, the patient continuously maintained clinical and biochemical remission, and endoscopic healing was observed at 1-year treatment with VDZ. One reason for this swift and favorable response to VDZ in this case report may be that the patient had not failed IFX due to poor response, but because discontinuation of IFX was inevitable due to active TB infection.
- Data regarding TDM in VDZ are limited. An exposure-efficacy relationship does not seem as straightforward as they are for anti-TNF agents, and robust target VDZ trough levels are not well-defined [18]. However, according to data from a TDM study in adult IBD, VDZ trough levels of >30 μg/mL at week 2, >24 μg/mL at week 6, and >14 μg/mL during maintenance therapy have been proposed for achieving clinical remission [19]. In another study in pediatric-onset IBD patients treated with VDZ, the mean VDZ trough level was 29.9 μg/mL at week 6 and 11.5 μg/mL during maintenance therapy [20]. In this case report, VDZ trough levels were lower than the proposed threshold targets; however, the patient responded well. This may be due to variability in the yet to be revealed association between individual pharmacokinetics and the degree of response to VDZ.
- In conclusion, we report a case of a pediatric patient with CD who successfully achieved and maintained remission with VDZ after developing active pulmonary TB during treatment with IFX. In cases that require recommencement of treatment with biologics after recovery of active pulmonary TB caused by anti-TNF agents, VDZ may be a good option even in pediatric IBD.
Discussion
-
Ethical statements
This study was approved by the Institutional Review Board (IRB) of Kyungpook National University Chilgok Hospital (IRB No: 2020-09-013). Informed consent was obtained from the patient and her parents.
-
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
-
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2017R1C1B5076980).
-
Author contributions
Conceptualization, Formal analysis, Investigation: SC, BK; Data curation: All authors; Funding acquisition, Methodology, Resources, Software, Supervision, Validation: BK; Project administration, Visualization: SC; Writing-original draft: SC; Writing-review & editing: BSC, BHC, BK.
Notes

- 1. Rosen MJ, Dhawan A, Saeed SA. Inflammatory bowel disease in children and adolescents. JAMA Pediatr 2015;169:1053–60.ArticlePubMedPMC
- 2. Kang B, Choe YH. Early biologic treatment in pediatric Crohn’s disease: catching the therapeutic window of opportunity in early disease by treat-to-target. Pediatr Gastroenterol Hepatol Nutr 2018;21:1–11.ArticlePubMedPMC
- 3. Kang B, Choi SY, Kim HS, Kim K, Lee YM, Choe YH. Mucosal healing in paediatric patients with moderate-to-severe luminal crohn's disease under combined immunosuppression: escalation versus early treatment. J Crohns Colitis 2016;10:1279–86.ArticlePubMed
- 4. Soler D, Chapman T, Yang LL, Wyant T, Egan R, Fedyk ER. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther 2009;330:864–75.ArticlePubMed
- 5. Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710.ArticlePubMed
- 6. Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013;369:711–21.ArticlePubMed
- 7. Turner D, Ruemmele FM, Orlanski-Meyer E, Griffiths AM, de Carpi JM, Bronsky J, et al. Management of paediatric ulcerative colitis, part 1: ambulatory care-an evidence-based guideline from European Crohn’s and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2018;67:257–91.ArticlePubMed
- 8. van Rheenen PF, Aloi M, Assa A, Bronsky J, Escher JC, Fagerberg UL, et al. The medical management of paediatric Crohn’s disease: an ECCO-ESPGHAN guideline update. J Crohns Colitis 2021;15:171–94.Article
- 9. Park DI, Hisamatsu T, Chen M, Ng SC, Ooi CJ, Wei SC, et al. Asian Organization for Crohn’s and Colitis and Asia Pacific Association of Gastroenterology consensus on tuberculosis infection in patients with inflammatory bowel disease receiving anti-tumor necrosis factor treatment. Part 1: risk assessment. Intest Res 2018;16:4–16.ArticlePubMedPMC
- 10. Park DI, Hisamatsu T, Chen M, Ng SC, Ooi CJ, Wei SC, et al. Asian Organization for Crohn’s and Colitis and Asia Pacific Association of Gastroenterology consensus on tuberculosis infection in patients with inflammatory bowel disease receiving anti-tumor necrosis factor treatment. Part 2: management. Intest Res 2018;16:17–25.ArticlePubMedPMC
- 11. Löwenberg M, Vermeire S, Mostafavi N, Hoentjen F, Franchimont D, Bossuyt P, et al. Vedolizumab induces endoscopic and histologic remission in patients with Crohn’s disease. Gastroenterology 2019;157:997–1006.ArticlePubMed
- 12. Colombel JF, Sands BE, Rutgeerts P, Sandborn W, Danese S, D’Haens G, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut 2017;66:839–51.ArticlePubMed
- 13. Abreu C, Magro F, Santos-Antunes J, Pilão A, Rodrigues-Pinto E, Bernardes J, et al. Tuberculosis in anti-TNF-α treated patients remains a problem in countries with an intermediate incidence: analysis of 25 patients matched with a control population. J Crohns Colitis 2013;7:e486–92.ArticlePubMed
- 14. Ledder O, Assa A, Levine A, Escher JC, de Ridder L, Ruemmele F, et al. Vedolizumab in paediatric inflammatory bowel disease: a retrospective multi-centre experience from the paediatric IBD porto group of ESPGHAN. J Crohns Colitis 2017;11:1230–7.ArticlePubMed
- 15. Singh N, Rabizadeh S, Jossen J, Pittman N, Check M, Hashemi G, et al. Multi-center experience of vedolizumab effectiveness in pediatric inflammatory bowel disease. Inflamm Bowel Dis 2016;22:2121–6.ArticlePubMed
- 16. Sands BE, Feagan BG, Rutgeerts P, Colombel JF, Sandborn WJ, Sy R, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology 2014;147:618–27.ArticlePubMed
- 17. Dulai PS, Boland BS, Singh S, Chaudrey K, Koliani-Pace JL, Kochhar G, et al. Development and validation of a scoring system to predict outcomes of vedolizumab treatment in patients with Crohn’s disease. Gastroenterology 2018;155:687–95.ArticlePubMedPMC
- 18. Pouillon L, Vermeire S, Bossuyt P. Vedolizumab trough level monitoring in inflammatory bowel disease: a state-of-the-art overview. BMC Med 2019;17:89.ArticlePubMedPMC
- 19. Dreesen E, Verstockt B, Bian S, de Bruyn M, Compernolle G, Tops S, et al. Evidence to support monitoring of vedolizumab trough concentrations in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2018;16:1937–46.ArticlePubMed
- 20. Aardoom MA, Jongsma MM, de Vries A, Wolthoorn J, de Ridder L, Escher JC. Vedolizumab trough levels in children with anti-tumor necrosis factor refractory inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2020;71:501–7.ArticlePubMed
References
Figure & Data
References
Citations

- The safety of vedolizumab in a patient with Crohn’s disease who developed anti-TNF-alpha agent associated latent tuberculosis infection reactivation: A case report
Yuya Sugiyama, Nobuhiro Ueno, Shion Tachibana, Yu Kobayashi, Yuki Murakami, Takahiro Sasaki, Aki Sakatani, Keitaro Takahashi, Katsuyoshi Ando, Shin Kashima, Kentaro Moriichi, Hiroki Tanabe, Toshikatsu Okumura, Mikihiro Fujiya
Medicine.2023; 102(28): e34331. CrossRef - Vedolizumab Is Safe and Efficacious for the Treatment of Pediatric-Onset Inflammatory Bowel Disease Patients Who Fail a Primary Biologic Agent
Sujin Choi, Eun Sil Kim, Yiyoung Kwon, Mi Jin Kim, Yon Ho Choe, Byung-Ho Choe, Ben Kang
Journal of Korean Medical Science.2022;[Epub] CrossRef

 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine PubReader
PubReader ePub Link
ePub Link Cite
Cite