Current perspectives in stem cell therapies for osteoarthritis of the knee
Article information
Abstract
Mesenchymal stem cells (MSCs) are emerging as an attractive option for osteoarthritis (OA) of the knee joint, due to their marked disease-modifying ability and chondrogenic potential. MSCs can be isolated from various organ tissues, such as bone marrow, adipose tissue, synovium, umbilical cord blood, and articular cartilage with similar phenotypic characteristics but different proliferation and differentiation potentials. They can be differentiated into a variety of connective tissues such as bone, adipose tissue, cartilage, intervertebral discs, ligaments, and muscles. Although several studies have reported on the clinical efficacy of MSCs in knee OA, the results lack consistency. Furthermore, there is no consensus regarding the proper cell dosage and application method to achieve the optimal effect of stem cells. Therefore, the purpose of this study is to review the characteristics of various type of stem cells in knee OA, especially MSCs. Moreover, we summarize the clinical issues faced during the application of MSCs.
Introduction
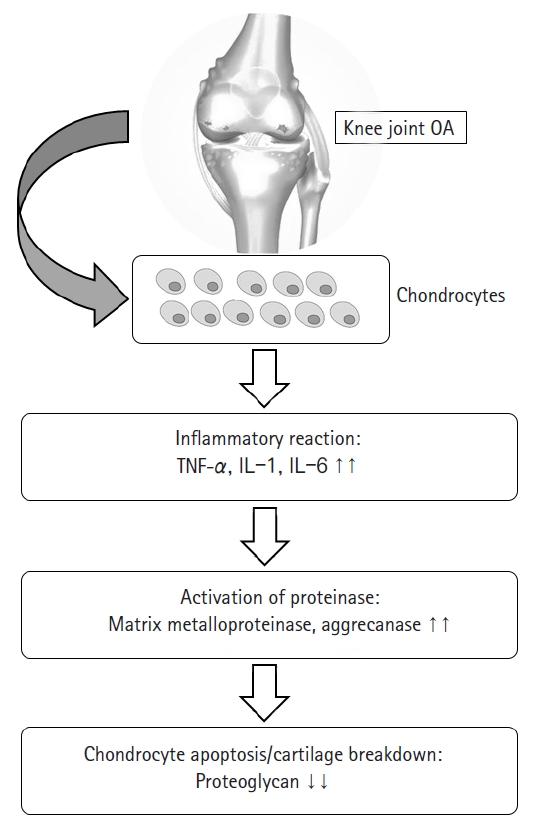
Osteoarthritis (OA) is a degenerative joint disease characterized by loss of cartilage, osteophyte formation, and periarticular bone change, resulting in disability [1,2]. In order to establish the disease-modifying strategies of OA, it is necessary to understand the biomolecular features seen in OA circumstance. Increased proinflammatory cytokines such as interleukin-1 or tumor necrosis factor-α, decreased growth factors such as transforming growth factor-beta (TGF-β), activated matrix metalloproteinase, and ultimate chondrocyte senescence can be observed at the molecular level [3-5] (Fig. 1). Although disease-modifying OA strategies that block inflammatory pathways and enhance cartilage protective function have been developed recently [6,7], their effects on preventing the progression of OA have been still unsatisfactory, and it is particularly difficult to achieve ultimate cartilage regeneration [8].
Meanwhile, since articular cartilage has a limited capacity for spontaneous healing, once damaged, it may eventually progress to OA [9]. Numerous attempts for the regeneration of focal cartilage defect have been made. Depending on the degree of defect size, various surgical options have been used, including multiple drilling, microfracture, abrasion chondroplasty, autologous chondrocyte implantation, and osteochondral autologous transplantation [10-13]. However, there has been no optimal regenerative method for cartilage in knee OA.
Recently, mesenchymal stem cells (MSCs) have been in the spotlight for their disease-modifying and chondrogenic potential along with their ease of harvesting, safety, and differentiation potential into cartilage [14,15]. Moreover, MSCs have been known to have paracrine and immunomodulatory effects through the secretion of cytokines and growth factors [16-19].
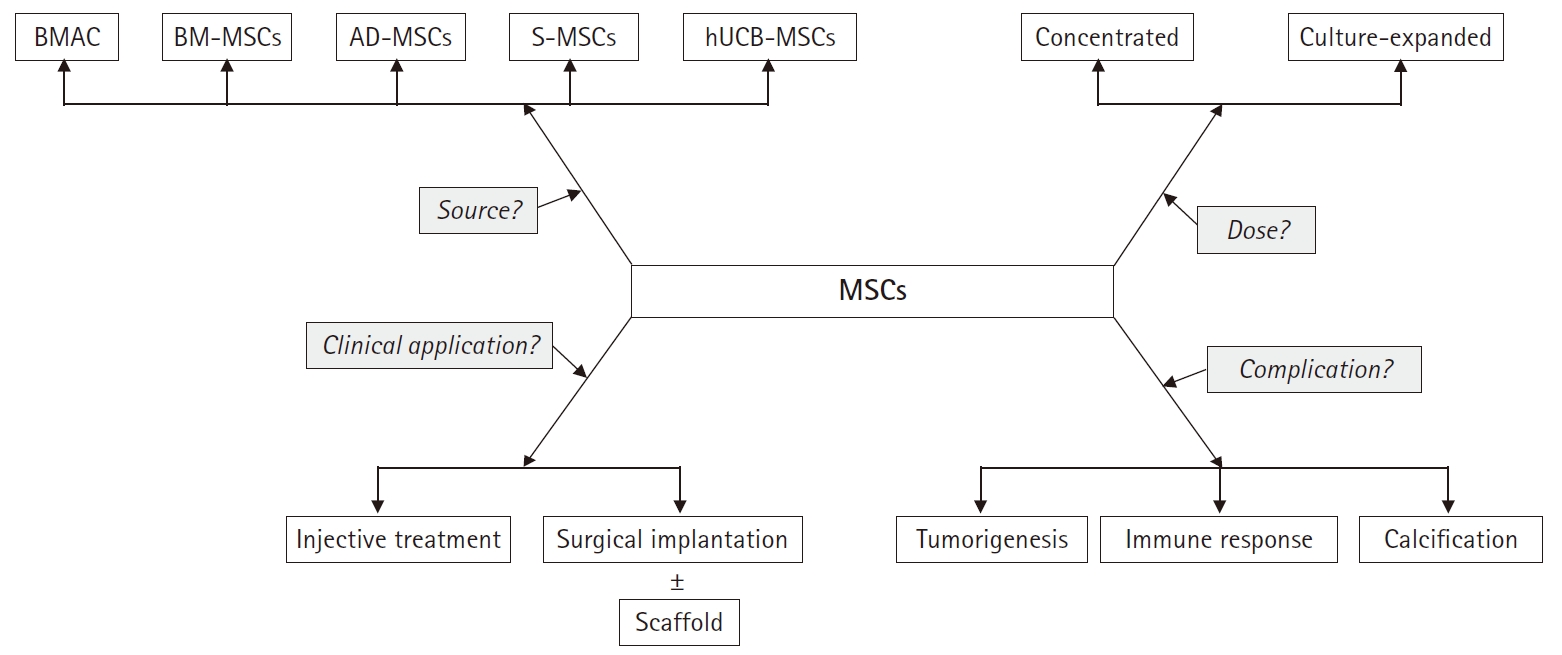
Therefore, considering the immunomodulatory and regenerative effect, stem cell therapies might be a promising line of treatment for knee OA. The purpose of this study was to review the characteristics of various type of stem cells in knee OA, especially based on MSCs. Moreover, we summarized the clinical issues for application of MSCs (Fig. 2).
General characteristics of stem cells
In general, stem cells can be divided into two major groups: (1) embryonic stem cells (ESCs) from the inner cell mass of blastocyst, with totipotency or pluripotency; (2) adult stem cells (tissue-specific stem cells) from specific organ, with multipotency [20,21]. Although adult stem cells have usually more restricted differentiation capacity compared to ESCs, they exhibit some advantages including safety, easy derivation, and tissue-specific differentiation potential [22]. Among them, MSCs appears to be the promising candidates.
MSCs are multipotent progenitor cells that can be obtained from bone marrow, adipose tissue, synovial membrane, and articular cartilage [23]. Several studies report their multidirectional differentiation potential [24,25]. Particularly, autologous MSCs can be easily harvested and applied in clinical settings, and allogenic cells can be utilized [14,19]. Culture expansion may be required to maximize their clinical effect [14], although that may result in functional deterioration, mutation, and tumorigenesis as passage progresses. Nevertheless, MSCs can be cultivated and amplified while maintaining their potential, and differentiated into a variety of connective tissues such as bone, adipose tissue, cartilage, intervertebral discs, ligaments, and muscles [26,27]. Owing to their inherent ability for self-renewal, proliferation, and differentiation toward mature tissues, MSCs could have promising applications in cell therapy and regenerative medicine [28]. The Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy proposed the definition of MSC via the following minimal set of criteria: (1) being plastic-adherent in standard culture conditions; (2) expressing CD105, CD73, and CD90 at their surface; while (3) lacking CD45, CD34, CD14, CD79α, and HLA-DR; and (4) being able to differentiate into osteoblasts, adipocytes, and chondrocytes in vitro [27].
Bone marrow-derived mesenchymal stem cells
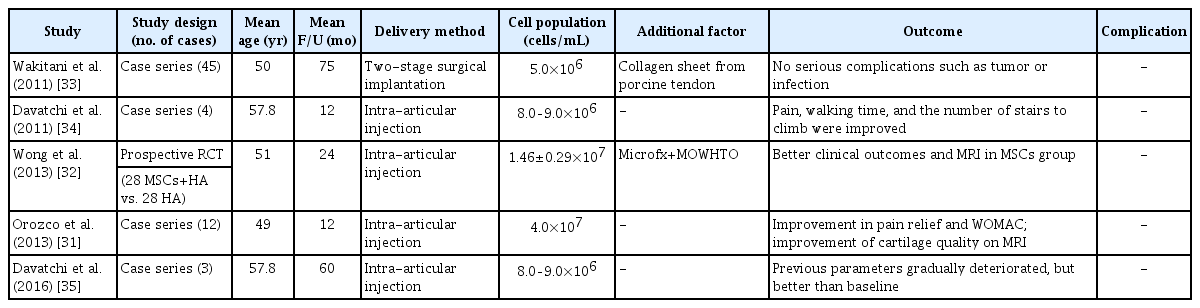
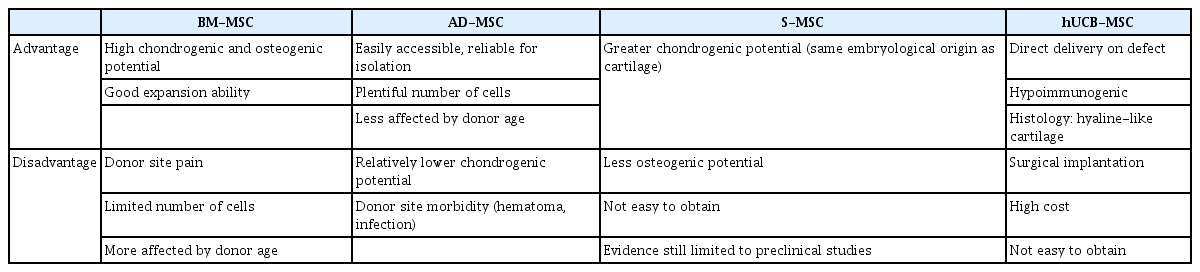
Bone marrow-derived mesenchymal stem cells (BM-MSCs) have been widely studied for OA treatment because of several advantages, including high expansion ability, and differentiation potential into cartilage [29-31]. Furthermore, due to easy harvesting from autologous bone marrow, they are cost-effective as compared to other types of MSCs. Several studies have reported favorable clinical outcomes in patients with knee OA who underwent intra-articular injection or surgical implantation using autologous BM-MSCs (Table 1) [31-35]. However, donor site pain, limited number of obtainable cells (0.001% of all nucleated cells in bone marrow), and decreased differentiation potential with increase in donor age are a few limitations while using BM-MSCs [31,36]. Bone marrow aspiration from anterior or posterior iliac crest can be performed with local anesthesia under ultrasonographic or fluoroscopic guidance to improve accuracy and efficiency [37].
Meanwhile, bone marrow aspirate concentrate (BMAC), which contains abundant nucleated cells and cytokines has been widely applied to the treatment of knee OA. It contains several factors involved in the healing process such as platelet-derived growth factor, TGF-β, vascular endothelial growth factor, and fibroblast growth factor [17,38]. BMAC can be easily extracted by via FDA approved commercialized kits and this process condenses the buffy coat containing mononuclear cells and increases the number of MSCs relative to baseline [39]. Recently, it is widely used in various orthopedic fields such as nonunion, osteonecrosis and sports injuries.
Centeno et al. [40] reported encouraging clinical outcomes with a low rate of adverse effects in autologous BMAC for intra-articular injection with or without adipose grafts in patients with knee OA. Additionally, significantly better results were observed in patients with Kellgren-Lawrence (K-L) grades I or II than in patients with K-L grades III or IV. Other studies have also reported positive clinical outcomes with simple intra-articular injections of BMAC [41,42]. Contrastingly, in a recently conducted prospective randomized controlled trial, Shapiro et al. [42] did not report encouraging outcomes of BMAC as compared to the control group. BMAC studies in OA patients have not yet shown consistent results. Further researches comprising well-designed, randomized, controlled trials with larger sample sizes are needed to elucidate the exact mechanism of BMAC.
Adipose tissue-derived mesenchymal stem cells
Adipose tissue, along with bone marrow, has been the most frequently used source for isolating MSCs [43]. Adipose tissue is abundant and easily accessible, making it a reliable site for stem cell isolation. It has copious numbers of MSCs (approximately 0.5×104–2.0×105/1 g fat) compared to BM-MSCs and their differential capacity is relatively less affected by donor age.
The specific premade solution is usually infiltrated into the subcutaneous tissue of the abdominal region through a tumescent technique, and then, a conventional abdominal liposuction is performed using blunt cannulas [44]. Enzymatic digestion of fat tissue is the most used isolation technique to obtain adipose tissue-derived mesenchymal stem cells (AD-MSCs) [45]. The extracted fat tissue is digested with enzyme (collagenase, dispase, or trypsin) to generate the stromal vascular fraction (SVF) that contains AD-MSCs and other endothelial and hematopoietic cells [46,47]. Among them, only the plastic-adherent, cultured and serially passaged multipotent cell populations are termed as AD-MSCs [48]. Meanwhile, non-enzymatic digestion, including mechanical procedures such as centrifugation and filtration is sometimes used. However, the range of yields shows high variation [49].
Several studies have reported encouraging clinical results for intra-articular injection of AD-MSCs in knee OA patients (Table 2) [14,50-53]. Lee et al. [50] reported that intra-articular injection of culture expanded AD-MSCs (1×108 cells) showed satisfactory functional improvement and pain relief in patients with knee OA through a prospective randomized controlled trial. Nonetheless, AD-MSCs have some disadvantages such as relatively lower chondrogenic potential and donor site morbidity [54].
SVF also can be obtained via enzymatic digestion and differential centrifugation of adipose tissue. SVF consists of a heterogeneous mesenchymal population of cells that includes not only adipose stromal and hematopoietic stem cells but also endothelial cells, erythrocytes, fibroblasts, lymphocytes, monocyte/macro-phages and pericytes (Table 3) [45,55]. Despite a highly heterogenous composition and low stem cell proportion, some studies have reported favorable clinical outcomes of SVF in knee OA due to their potential and ease of use [56,57].
Synovium-derived mesenchymal stem cells
Synovium-derived mesenchymal stem cells (S-MSCs) have attracted considerable attention due to their high chondrogenic potential and less hypertrophic differentiation than BM-MSCs [58,59]. Embryologically, S-MSC-derived chondrocytes and articular chondrocytes share similar gene expression profile [60]. They may prove to be the optimal cell source of MSCs as native nature to the joints. Kubosch et al. [58] reported that S-MSCs play an important role in joint homeostasis and possibly in natural cartilage repair. However, most of their evidence was limited to preclinical studies [61]. Only one retrospective study has reported the results of S-MSCs in human OA of the knee joint [62]. They reported clinical improvement and secure defect filling confirmed using second-look arthroscopy and magnetic resonance image, 48 months postoperatively. Further researches are needed to elucidate the interaction of S-MSCs and chondrocytes, and the promising role of S-MSCs in cartilage tissue engineering.
Human umbilical cord blood-derived mesenchymal stem cells
Umbilical cord compartments including Wharton’s jelly, perivascular tissue, and umbilical cord blood (UCB) can be utilized to isolate MSCs [43,63]. Umbilical cord-derived MSCs can be obtained through pain-free collection methods with fewer ethical issues. An experimental comparative study [64] confirmed that UCB-MSCs have biological advantages in comparison to bone marrow or adipose tissue, including higher rate of proliferation and clonality, retardation of senescence, and superior anti-inflammatory effect.
Recently, clinical outcomes of human UCB-MSCs (hUCB-MSCs) for cartilage regeneration have been reported [65-67], and their medicinal product mixed with hyaluronic acid (Cartistem; Medipost, Seongnam, Korea) has been widely applied in clinical settings. hUCB-MSCs are also isolated in a non-invasive manner and have the advantage of being hypoimmunogenic. Moreover, they show a hyaline-like histological morphology [67]. Park et al. [65] reported that an hUCB-MSC-based product appeared safe and effective for the regeneration of hyaline-like cartilage in OA of the knee after 7 years of follow-up. In our institution, commercialized hUCB-MSCs were performed on OA of the knee to obtain favorable clinical outcomes and highly qualified regeneration (Fig. 3).
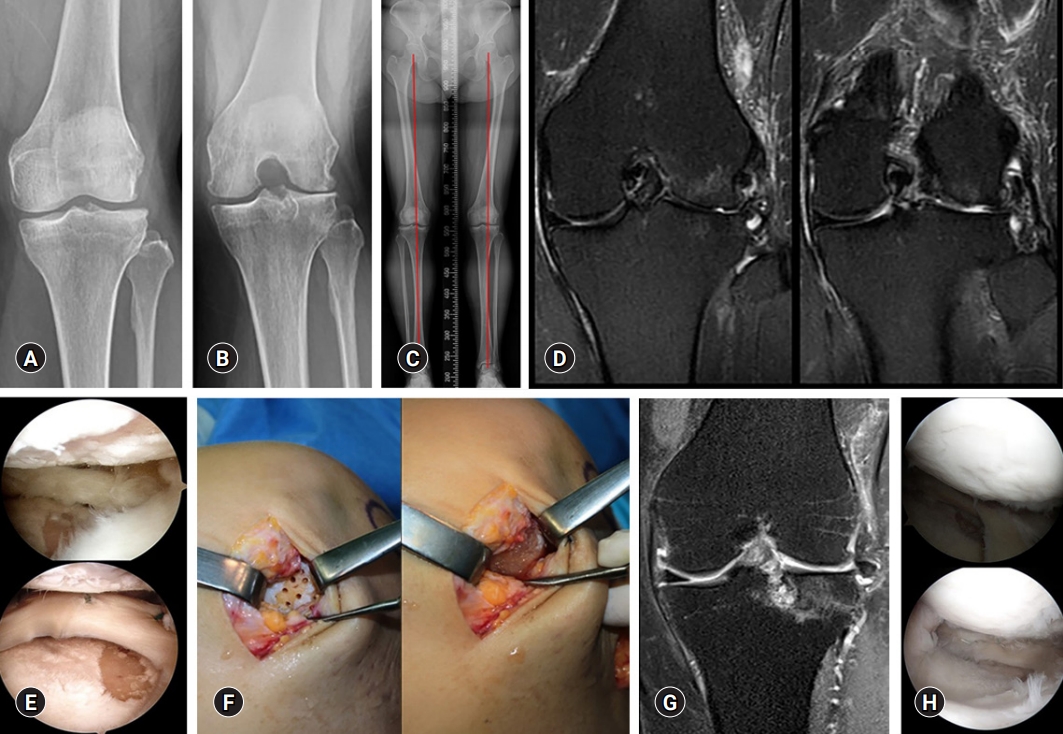
(A, B) Kellgren-Lawrence grade III osteoarthritis is observed in the left knee anteroposterior and 45° flexion standing radiographs in a 49-year-old woman. (C) The scanography image shows neutral alignment of the both lower extremities. (D, E) Coronal T2-weighted fat suppressed magnetic resonance image (MRI) and arthroscopy show the focal chondral defects of International Cartilage Repair Society (ICRS) grade IV in the lateral femoral condyle and lateral tibial plateau. (F) Surgical implantation is performed using a commercialized mixture of human umbilical cord blood-derived mesenchymal stem cells and hyaluronic acid gel (Cartistem). (G, H) The coronal T2-weighted fat suppressed MRI and second-look arthroscopy confirm regenerated cartilage at 18 months after surgery.
Clinical issues for application of mesenchymal stem cells
There is no consensus on the optimal dose or cell number to achieve the utmost effect of stem cells. The optimal dose of MSC implantation for cartilage regeneration has not yet been established. In a dose-dependent prospective study, Jo et al. [14] reported that significant clinical improvement was shown only in the high dose group (1×108 cells). Based on this result, culture expansion may be needed to obtain the optimal effect of MSCs.
Treatment strategies for clinical application may also be one of the issues faced by clinicians. Injective treatment is relatively efficient because it is easy to apply and does not require hospitalization, but precise delivery to target site may be difficult [54]. Conversely, surgical implantation allows direct delivery to the lesion site, but requires hospitalization and is a more invasive approach. Another option is to mix MSCs with biodegradable scaffolds followed by surgical implantation. The three-dimensional scaffold maintains the phenotype of differentiated chondrocytes, promotes improved chondrogenesis through uniform cell distribution, and reduces the risk of chondrocyte leakage [68]. Scaffold materials include hyaluronic acid, collagen derivatives, agarose, fibrin glue, and chitosan [69]. However, chondrocyte dedifferentiation, apoptotic cell leakage, inadequate cell distribution, and low differentiation have been reported in scaffold-based studies [70].
Potential risks of MSCs in clinical use, such as tumorigenesis, immune response, and heterotrophic calcification are also considerable issues [71]. Therefore, it should be recognized that such risk of MSC-mediated abnormal reactions might occur in some cases, and mandating a careful assessment of the patient's condition. Further research is also needed to guarantee the safety of MSCs. Each type of MSCs is summarized in Table 4.
Other advanced techniques
1. Induced pluripotent stem cell
Induced pluripotent stem cells (iPSCs) are also becoming a promising cell source for stem cell-based therapy [72]. They are a kind of stem cells established in the laboratory that can be reprogrammed into somatic cells. Although therapeutic models of neurological and cardiovascular diseases using iPSCs have been reported [73,74], the research using iPSCs in orthopedic fields is still in its nascent stages, particularly for cartilage regeneration. iPSCs exhibit similar proliferation capacity and pluripotency as other tissue-derived stem cells, with no immune rejection and ethical issues [72]. Recently, new methods for producing iPSCs without viral vectors to reduce the risk of tumorigenicity have been developed [75]. Nonetheless, to date, limited data exists regarding the in vitro chondrogenic differentiation of iPSCs and the yield of iPSCs is relatively low.
2. Genetically modified MSCs (engineered MSCs)
The efficacy of MSCs in vivo may still be low due to poor survival, retention, and engraftment of the cells. Most MSCs often die within the first few hours after in vivo delivery [76]. Therefore, MSCs need to be genetically modified to improve survival, migration, and secretion of growth factors for their application in regenerative medicine [76]. Genetic modification of MSCs is usually achieved through viral vectors [76]. The most commonly used vectors include retrovirus, lentivirus, baculovirus, and adenovirus [22]. Viral transduction has improved homing of MSCs to the defect or inflammation site through the overexpression of homing receptors. MSCs have been transduced with adenovirus expressing C-X-C chemokine receptor 4 (CXCR-4) and runt-related transcription factor-2 (Runx-2), and with retrovirus expressing receptor activator of nuclear factor-kB and CXCR-4 [77,78]. Although the efficacy of genetically modified MSCs has been demonstrated in preclinical studies, it has not been investigated in clinical trials.
Conclusion
MSCs are the hottest topic in recent stem cell research. The application of stem cells in cartilage regeneration has been tried a lot, but so far, the effect of cartilage regeneration is not consistent from one study to another. Moreover, the most appropriate cell source is still controversial. Further research is needed to determine which tissue-derived stem cells, which usage and dose will be ideal for the treatment of osteoarthritis. In this review, we briefly reviewed the most up-to-date knowledge, including the characteristics, types, and clinical issues of MSCs. It is expected that in future, treatment with MSCs will be applied more clinically in the treatment of knee OA.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: GBK, OJS; Data curation: GBK; Formal analysis: GBK; Methodology: GBK; Project administration: OJS; Investigation, Resources: GBK; Supervision: OJS; Validation: GBK; Writing-original draft: GBK; Writing-review & editing: GBK, OJS.