Clinical significance of lymph node size in locally advanced cervical cancer treated with concurrent chemoradiotherapy
Article information
Abstract
Background
This study aimed to assess the in-field lymph node (LN) failure rate according to LN size and to investigate effect of LN size on the survival outcome of patients with locally advanced cervical carcinoma treated with concurrent chemoradiotherapy (CCRT).
Methods
A total of 310 patients with locally advanced cervical carcinoma treated with CCRT were enrolled in retrospective study. LN status was evaluated by magnetic resonance imaging. All patients received conventional external beam irradiation and high-dose rate brachytherapy, and concurrent cisplatin-based chemotherapy. In-field LN failure rate according to LN size was analyzed.
Results
The median follow-up period was 83 months (range, 3-201 months). In-field LN failure rate in patients with pelvic LN size more than 10 mm was significantly higher than that in patients with pelvic LN size less than 10 mm (p<0.001). A similar finding was observed in the in-field para-aortic LN (PALN) failure rate (p=0.024). The pelvic and PALN size (≥10 mm) was a significant prognostic factor of overall-survival (OS) and disease-free survival rate in univariate and multivariate analyses. The OS rate was significantly different between groups according to LN size (<10 mm vs. ≥10 mm).
Conclusion
A LN of less than 10 mm in size in an imaging study is controlled by CCRT. On the other hand, in LN of more than 10 mm in size, the in-field LN failure rate increase and the prognosis deteriorate. Therefore, a more aggressive treatment strategy is needed.
Introduction
Cervical cancer is the fourth most common cancer worldwide with an annual mortality rate of 250,000 in developing countries [1]. Since 1999, five phase III randomized clinical trials reported significant survival advantages for patients who received concurrent chemoradiotherapy (CCRT) compared with those who received radiotherapy alone [2]. CCRT has become the standard treatment for locally advanced cervical cancer [3].
The incidence of lymph node (LN) involvement in locally advanced cervical cancer is 39-44% and the incidence of para-aortic LN (PALN) involvement is approximately 8-16% [4-6]. Radiotherapy for metastatic regional LNs is not well established in cervical cancer. Recent study about radiation field failure after definitive CCRT in patients with locally advanced cervical cancer (stage IB-IVA) reported that the estimated 3-year rate of locoregional control was about 89% [7]. There are variables that affect in-field failure, such as tumor size (>5 cm), young age (<40 years), non-squamous histology and positive LN [7]. It is assumed that control of metastatic regional LNs will be of clinical significance in patients who do not have local failure. The staging of cervical cancer follows the International Federation of Gynecology and Obstetrics (FIGO) stage system based on clinical staging. Although LN metastasis serves an important role in prognosis, LN status is not included in the staging [8]. In general, the larger the size of the primary tumor, the greater the dose required for achieving tumor control [9-11]. Similarly, the dose required to achieve local control of a metastatic LN increases with the size of the metastasis. The larger the size of the LN, the poorer the prognosis and local control rate. In many institutions, external beam boost irradiation has been used empirically for large metastatic LNs. However, there is limited clinical data for external beam irradiation to metastatic LNs [12-15], and the association between the size of the metastatic LN and LN control in CCRT remains to be fully elucidated. Previous studies included mixed groups comprising patients treated with CCRT or radiotherapy alone, which is inadequate to validate the effect of CCRT. Therefore, we aimed to assess the in-field LN failure rate according to LN size and to investigate the effect of LN size on the survival outcome in patients with cervical cancer treated with CCRT.
Materials and methods
1. Patients
This retrospective study was approved by the Institutional Review Board in Daegu Catholic University Medical Center (IRB No. CR-17-045-L). The medical records of 335 patients with cervical cancer treated with CCRT at the Daegu Catholic University Medical Center between 2000 and 2016, were reviewed. The inclusion criteria were as follows: 1) newly diagnosed histologically proven squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma of the uterine cervix; 2) treatment using platinum-based CCRT; and 3) clinical and radiologic FIGO stage IB-IVA with no other evidence of distant metastasis. Of 335 patients, 25 patients were excluded for the following reasons: 1) surgical intervention prior to CCRT (n=20); and 2) incomplete treatment (n=5). The remaining 310 patients were included in the analysis. LN metastasis was evaluated by magnetic resonance imaging (MRI).
A total of 142 patients (45.8%) had metastatic pelvic LNs and 17 patients (5.5%) had metastatic PALNs. To investigate the effect of LN size on the treatment outcome, the patients were divided based on the LN size regardless of MRI evaluation results: those who had pelvic LN size less than 10 mm (n=196), those who had pelvic LN size from 10 mm to 19.99 mm (n=90), and those who had pelvic LN size 20 mm or more (n=24). Further, 189 patients had PALN size less than 5 mm, 108 patients had PALN size from 5 mm to 9.99 mm and 13 patients had PALN size 10 mm or more. Patients’ characteristics are shown in Table 1.
2. Evaluation of lymph node status
The LN status was evaluated mainly by MRI. The LN status was assessed using a combination of size, shape, and internal architecture [16,17]. For determining the LN size, the short axis diameter of the largest LN was measured. At our institution, positron emission tomography/computed tomography (PET/CT) has been in use since 2005. For evaluating a metastatic LN, PET/CT shows better accuracy than MRI. However, it cannot change the prognosis [18]. Therefore, we use mainly MRI rather than PET/CT for evaluating LN status.
3. Treatment
All patients were scheduled to receive combined external-beam radiotherapy (EBRT) and intracavitary brachytherapy (ICBT). Seventy-two patients received extended-field pelvic radiotherapy (EF-PRT), and the superior border was extended to encompass the PALN area. In the patients with PALN involvement (n=17), PALN irradiation was done. In patients with no evidence of PALN involvement (n=55), the decision to use EF-PRT was at the discretion of the radiation oncologist, balancing the risk of occult PALN metastases against the potential for increased acute and late toxicity. In EF-PRT, the superior border was extended to encompass PALN area according to the discretion of the radiation oncologist as follows: T12-L1 (n=20), L1-L2 (n=5), or L2-L3 (n=47) interspace. All patients received a median EBRT dose of 45 Gy (range, 39.6-54 Gy) at 1.7 Gy (in some EF-PRT cases only) or 1.8 Gy per fraction with whole pelvic radiotherapy (WPRT) or EF-PRT. After WPRT or EF-PRT, the boost irradiation of median 9 Gy (range, 5.4-23.4 Gy) given at 1.8 Gy or 2 Gy per fraction to LN regions that had significant evidence of carcinoma involvement or LN more than 10 mm on MRI findings, involved parametrium, or involved regions of the pelvic sidewall. In boost irradiation, three-dimensional conformal radiotherapy or intensity-modulated radiotherapy (IMRT) has been used since 2009. After adequate tumor regression, high-dose-rate ICBT was performed twice per week using an iridium-192 remote after-loading technique. The standard prescribed dose for each brachytherapy in our institution was 5.0 Gy to A-point in six fractions, twice weekly. The prescribed A-point dose was median 30 Gy (range, 15-36 Gy). The combined total dose from EBRT and ICBT was calculated using a linear quadratic model to determine the radiobiological equivalent dose in 2 Gy fractions (EQD2) (α/β=10) [19]. The median total prescribed EBRT EQD2 to pelvic LNs area and PALN area was 53.1 Gy (range, 44.25-69.03 Gy) and 44.25 Gy (range, 40.71-58.41 Gy). The median total prescribed A-point EQD2 (EBRT+ICBT) was 81.75 Gy (range, 69.36-105.70 Gy). The median overall irradiated time was 59 days (range, 45-133 days; interquartile range, 54-63 days).
All patients received radiotherapy and concurrent cisplatin-based chemotherapy. During radiotherapy, chemotherapy with weekly cisplatin (40 mg/m2 weekly for 6 weeks) was given to 200 patients. Two cycles of cisplatin-based combination chemotherapy with cisplatin plus 5-fluorouracil (5-FU), or cisplatin plus paclitaxel at 3 weeks intervals during external beam radiotherapy were given to 52 and 58 patients, respectively. Chemotherapy with cisplatin and 5-FU consisted of an intravenous infusion of 75 mg/m2 of cisplatin (day 1), followed by an intravenous infusion of 4,000 mg/m2 of 5-FU over a 96-hour period (days 2-5). One liter of normal saline was given before and after cisplatin, and mannitol was used to increase the urine output (day 1). Chemotherapy with cisplatin plus paclitaxel consisted of an intravenous infusion of 135 mg/m2 of paclitaxel (day 1), followed by an intravenous infusion of 75 mg/m2 of cisplatin (day 2).
4. Response evaluation and follow-up
All patients were subjected to routine post-CCRT surveillance with physical examination, cervicovaginal cytology, laboratory test (e.g., squamous cell carcinoma antigen), and imaging studies, including abdominopelvic CT, MRI, and PET/CT. After completion of CCRT, the patients were evaluated every 3 months for the first 2 years and every 6 months thereafter. Recurrence was diagnosed through physical examination and diagnostic imaging (contrast-enhanced CT, MRI, and/or PET/CT scans) [16,17] and was confirmed histologically via needle aspiration or excisional biopsy when possible.
5. End points and statistical methods
The primary endpoint was in-field LN failure rate according to the size of LNs, and the overall survival (OS) rate and disease-free survival (DFS) rate according to the size of LNs. LN failure within the irradiated region was considered an in-field LN failure. We calculated all occurrences from the date of diagnosis to the date of relapse or the last date of follow-up. Deaths from other cause were censored at the time of last follow-up.
Comparison of variables was based on the t-test. The survival analysis was based on the life-table method of Kaplan-Meier. Univariate analyses were performed with log-rank tests. The Cox proportional hazard model was used to construct a multivariate model to predict survival. p-values were the result of two-sided tests and p-value <0.05 was considered statistically significant. Statistical analysis was performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
Results
1. Analysis of lymph node size
The total number of patients was 310. Patients were divided into four groups according to pelvic LN status and size evaluated by MRI. Of these, 168 patients had LNs that had no significant evidence of carcinoma involvement on MRI, and had a short axis of less than 10 mm (group 0). The other 142 patients had LNs with evidence of heavily involved carcinoma on MRI, and, these patients were further divided into three groups according to their LN size (group 1, <10 mm; group 2, 10-19.99 mm; group 3, ≥20 mm).
On evaluation the in-field failure rate for the PALN area, 72 patients who received EF-PRT were analyzed. These patients were divided into three groups according to their PALN size (group 0, <5 mm; group 1, 5-9.99 mm; group 2, ≥10 mm).
2. In-field pelvic lymph node failure rate
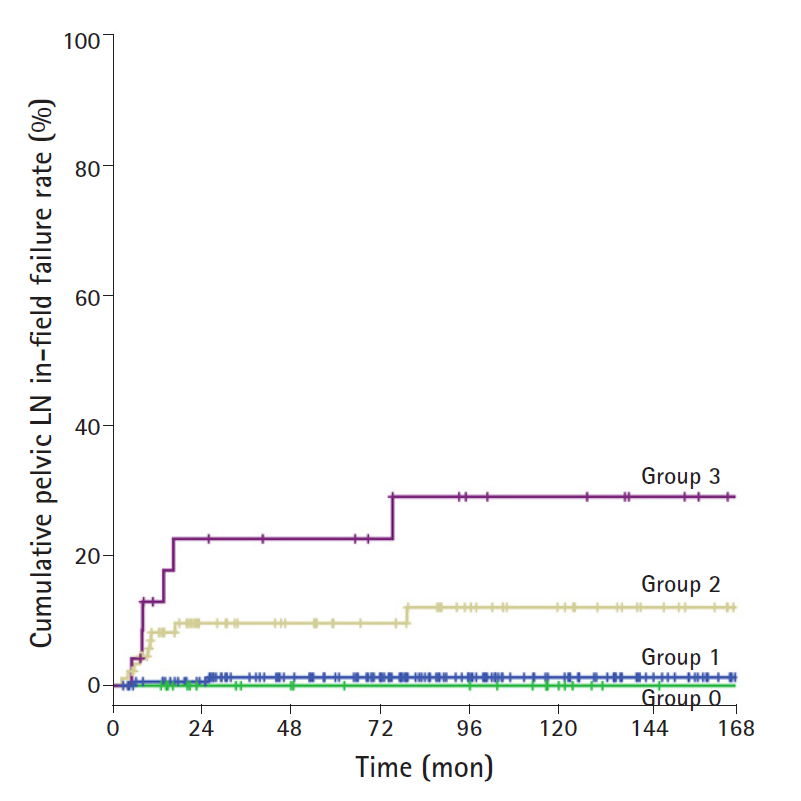
Due to the possibility of micro-metastasis of LN, the failure rate of all groups was analyzed. There was no significant difference between group 0 and group 1 (5-year in-field failure rate, 1.3% and 0%, respectively; 10-year in-field failure rate, 1.3% and 0%, respectively). The 5-year in-field failure rates among the patients in the groups 1, 2, and 3 were 0%, 9.6%, and 22.6%, respectively. The 10-year in-field failure rates among the patients in the groups 1, 2, and 3 were 0%, 12%, and 29%, respectively. There were statistically significant differences in the 5- and 10-year in-field failure rates between group 1 and group 2/3 (group 1 vs. group 2, p<0.001; group 1 vs. group 3, p<0.001). The in-field failure rate in patients with LN size 10 mm or more was significantly increased. In addition, although there was no statistically significant difference, the in-field failure rate tended to increase as the size increased (group 2 vs. group 3, p=0.089). The cumulative in-field pelvic LN failure rate according to LN size is shown in Fig. 1.
3. In-field para-aortic lymph node failure rate
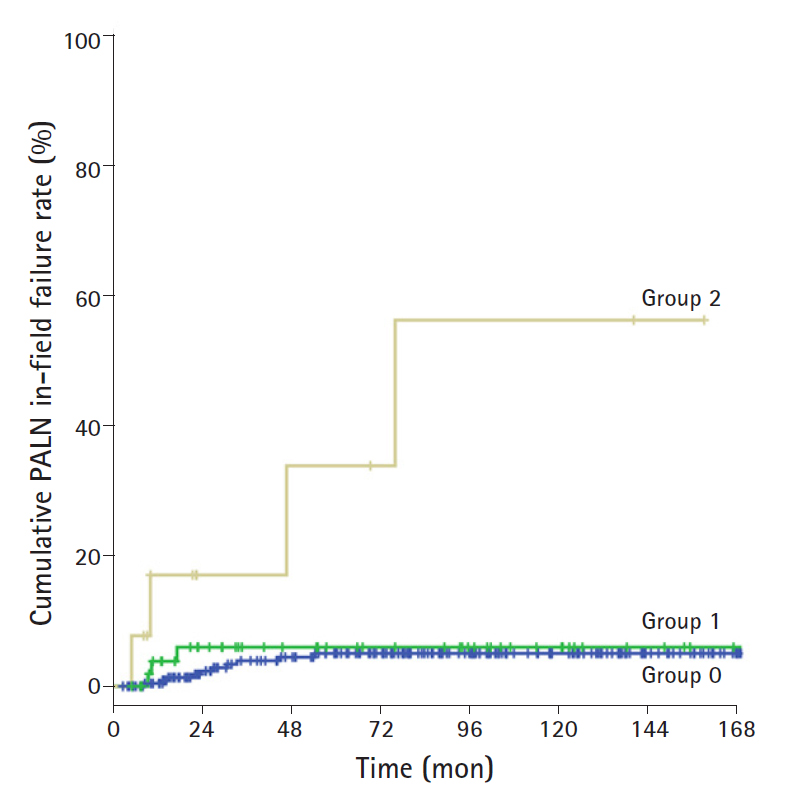
The 5-year in-field failure rates among patients in groups 0, 1, and 2 were 5.9%, 5%, and 18.5%, respectively, and 10-year in-field failure rates were 5.9%, 5%, and 45.7%, respectively. There were statistically differences between group 0/1 and group 2 (group 0/1 vs. group 2, p=0.024). Like the in-field failure rate for pelvic LN, the in-field failure rate for PALN was significantly increased with LN size 10 mm or more. The cumulative in-field PALN failure rate according to LN size in patients treated with EF-PRT is shown in Fig. 2.
4. Survival outcome: prognostic variables
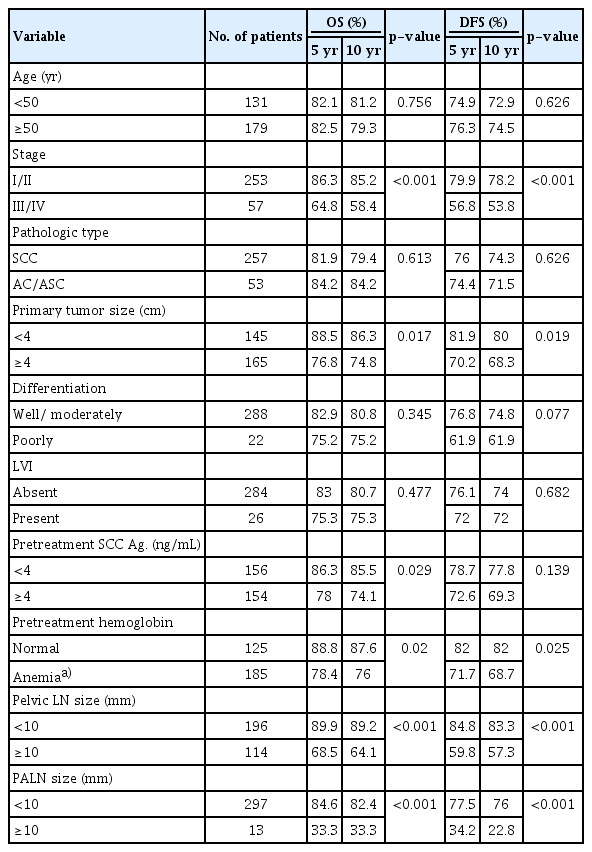
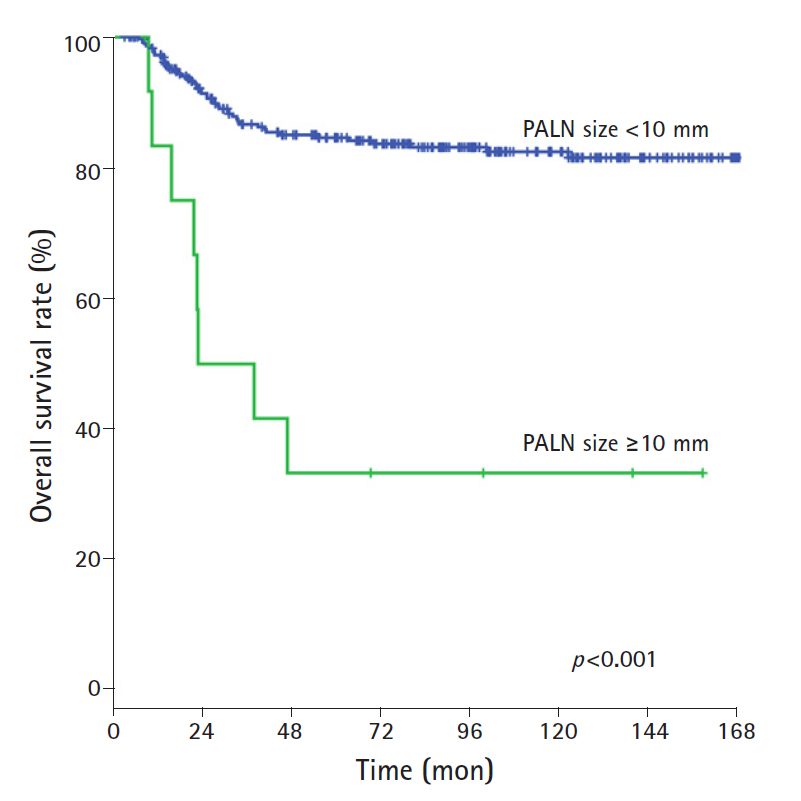
The results of the univariate analysis showed that advanced stage (I/II vs III/IV), pretreatment hemoglobin (<12.3 g/dL), tumor size (≥ 4 cm), and LN size in the pelvic and para-aortic areas (≥10 mm) were significant factors of poor OS rate and DFS rate. The 10-year OS rate in patients with pelvic LN size <10 mm and ≥10 mm was 89.2% and 64.1%, respectively, and the 10-year OS rate in patients with PALN size <10 mm and ≥10 mm was 82.4% and 33.3%, respectively (Table 2). The OS rates were statistically different according to LN size 10 mm or more for both pelvic and PALNs (pelvic LN, p<0.001; PALN, p<0.001; Figs. 3, 4). The 10-year DFS rate in patients with pelvic LN size <10 mm and ≥10 mm was 83.3% and 57.3%, respectively, and the 10-year DFS rate in patients with PALN size <10 mm and ≥10 mm was 76.0% and 22.8%, respectively. Of these, pelvic and PALN size (≥10 mm) was a significant prognostic factor of OS and DFS rates in multivariate analysis (pelvic LN, p=0.003; PALN, p=0.033, Table 3). The irradiated dose was not significantly associated with poor OS and DFS rates.
Discussion
Although standard radiotherapy regimens have been established for the treatment of primary cervical cancer, optimal radiotherapy regimens for regional LN metastases remain unclear, particularly for bulky LN [20,21]. The relationship between the size of metastatic LN and LN control in CCRT remains to be fully elucidated. In our study, LN size less than 10 mm was well-controlled, and the in-field failure rate for LN sizes ≥10 mm was increased. The in-field failure rate tended to increase as the LN size increased.
In the era of radiotherapy alone for advanced cervical cancer, Hacker et al. reported that the surgical removal of enlarged LNs prior to radiotherapy improves prognosis [22]. After the introduction of CCRT, since chemotherapy acts as a radiosensitizer, it may affect the control rate after bulky LN dissection, however, the results are insufficient. Lai et al. reported a study that assessed the prognostic significance of surgical staging in locally advanced cervical cancer [23]. The progression-free survival and OS rates of patients who underwent surgical staging were significantly poorer than those of patients who underwent clinical staging. It was suggested that the surgical assessment of PALN status prior to CCRT was ineffective compared with the use of imaging techniques [23-25]. Because the benefits of surgical dissection and biopsy are unclear, in the present clinical setting, oncologists use a radiologic method for the evaluation of LN status in almost all cases of locally advanced cervical cancer.
Therefore, currently, it is universally accepted that CCRT be performed following imaging studies in patients with locally advanced cervical cancer. The preoperative PET/CT evaluation of LNs assists in identifying distant metastasis and PALN metastasis, but dose not appear to improve survival rate. On evaluation of LN metastasis assessed by MRI, a wide range of sensitivity, specificity, and accuracy were reported; 30-73%, 93%, and 73%, respectively [26,27]. In addition, it has been suggested that the probability of microscopic LN disease is 20-25%, even with no significant evidence of carcinoma involvement in an imaging study [28]. However, in our study, the in-field failure rate and OS differed only according to the LN size despite the limitation of clinical staging. For pelvic LNs, there was no significant difference between group 0 and group 1 (5-year in-field failure rate, 1.3% and 0%, respectively; 10-year in-field failure rate, 1.3% and 0%, respectively). Therefore, for an LN size of less than 10 mm, the rate of control is similar regardless of whether the MRI reveals have significant evidence of carcinoma involvement. Negative LNs that had no significant evidence of carcinoma involvement or LNs less than 10 mm in MRI findings showed good control over conventional radiation dose, regardless of the sensitivity. Not all enlarged LNs are metastatic; however, they have a high metastatic potential. In our study, the factor of LN size ≥10 mm was associated with an increase in the in-field failure rate and affected the survival outcome confirmed by multivariate analysis. Therefore, it is appropriate to use LN size measurement MRI in treatment planning.
There are several studies assessing the effects of LN size on prognosis. Studies have reported that an enlarged LN, based on 20 mm or 15 mm size, is associated with a poor prognosis [15,29,30]. Song et al. reported that the OS and DFS rate were poorer when the LN size was larger than 1.5 cm [15]. Since the study included mixed groups composed of patients treated with CCRT or radiotherapy alone, it is inadequate to validate the effect of CCRT. In the present study, the size of 10 mm, which is suspicious of imaging positive lymphadenopathy, was used as the most commonly used size criteria. As a result, it was confirmed that an LN size ≥10 mm affects the in-field failure rate and OS rate in a CCRT setting [31]. There was no significant difference in the in-field failure rate (p=0.068) or OS (p=0.525) when a pelvic LN ≥20 mm was compared to a pelvic LN size ≥10 mm. Therefore, it is reasonable to use 10 mm as a criterion for determining positive LN status.
Optimal treatment with CCRT for an enlarged LN in locally advanced cervical cancer remains controversial. Traditionally, doses of 45-50 Gy in conventional fractionation are delivered to the whole pelvis to treat cervical cancer with or without concurrent chemotherapy, primarily due to adjacent normal tissue tolerance as a limiting factor. Subsequent small field radiation boosts of 5.4-10 Gy in conventional fractionation are frequently administered to metastatic LNs. The interdigitation pelvic node boosts with brachytherapy can present with specific challenges. An anteroposterior-posteroanterior boost technique may be used if boost fields are small and if less than an additional 5.4-10 Gy is needed to increase the combined external beam and brachytherapy dose to a minimum of 60-66 Gy [20,21]. According to Toita et al., the prognosis was no poorer when the LN was irradiated with a dose less than that given using the conventional method [32]. Recently, Ariga et al. demonstrated that external beam boost irradiation to positive pelvic LNs achieves favorable nodal control without increasing late complications [13]. Hata et al. also reported the radiotherapy effectively controlled pelvic LN metastases in patients with cervical cancer with most LNs <24 mm in diameter controlled by the total dose of 50.4 Gy in 1.8 Gy fractions and radiation boost over 50.4 Gy may improve the control of metastatic LNs ≥24 mm, particularly with concurrent chemotherapy [12]. They also suggested that higher doses to metastatic LNs may increase intestinal toxicities. In this CCRT study, the median total prescribed EBRT EQD2 to pelvic LNs area and PALN area was 53.1 Gy (range, 44.25-69.03 Gy) and 44.25 Gy (range, 40.71-58.41 Gy). It considered that in our study, pelvic LNs and PALNs that had evidence of heavily involved with carcinoma on MRI was given the dose as a traditional standard with concurrent chemotherapy. Despite the traditional standard dose in CCRT, our study showed that significantly higher incidence of in-field LN failure LNs recurrence in patients with pelvic LN size ≥10 mm, than that in patients with pelvic LN size <10 mm. Therefore, the development of more effective radiotherapy strategies is required to reduce the pelvic LN recurrence in patients with pelvic LN size ≥10 mm.
A higher dose than the traditional standard dose can be delivered using additional boost technique. Hata et al. reported that larger LNs that were >24 mm in diameter may require higher doses, up to about 55.8 Gy [12]. Rash et al. reported that the control rate was improved when a total dose of ≥54 Gy was delivered using a boost technique to treat pelvic and para-aortic lymphadenopathy [33]. However, results on the toxicity associated with higher radiation doses are insufficient. Normal tissue complication probability should be considered when increasing the radiation dose for achieving control of an enlarged LN. High-dose boost irradiation to enlarged LNs may increase the risk of high-dose exposure to the colon and small intestine due to their proximity to pelvic LNs. When higher boost doses are required, more complex techniques are recommended; however, to avoid compromising subsequent brachytherapy, care must be taken to minimize the dose to the bowel, rectum, and bladder from high-precision radiotherapy such as image-guided radiotherapy (IGRT) and IMRT. Recently, dose escalation studies have been performed using IMRT, volumetric modulated arc therapy, and IGRT; however, the problem of bowel toxicity remains, which limits the use of higher irradiation doses [34-36]. There are problems with the IMRT itself which are related to target definition, inter- and intra-fraction motion, and tumor regression during treatment [37,38]. However, some studies have shown that excellent control of the metastatic LNs with a median dose of 62 Gy (range, 59.4-64 Gy) using IMRT was achieved. Thus, we need to wait for the results of further randomized prospective trials and the result of long-term follow-up studies. Additionally, the use of high-precision radiotherapy such as image-guided stereotactic body radiotherapy or particle radiotherapy is expected to be beneficial for boost irradiation to enlarged LNs. By using these recent advanced treatment methods, higher doses can be delivered to the tumor without increasing doses to adjacent normal tissue.
A limitation of the present study includes its retrospective study design, which may be affected by selection bias. The LN dose by ICBT was not analyzed as image-guided brachytherapy was not performed, and irradiation dose to pelvic LNs may have been underestimated. There was a relatively small number of patients with PALNs of ≥10 mm. Therefore, caution is required when ascribing clinical meaning to the results of the present study. The results of the present study must be validated on a larger patient cohort and further prospective randomized investigations of radiation dose escalation with IMRT are required to decrease pelvic LN recurrence.
In conclusion, the present study demonstrates that an LN of less than 10 mm in size in an imaging study was controlled by CCRT. On the other hand, in CCRT with boost irradiation to LNs of size ≥10 mm, the in-field failure rate increases, and the prognosis deteriorates. Currently, treatment guidelines for enlarged LNs remain unclear; therefore, a more aggressive treatment strategy to overcome the adverse effects of enlarged LNs on survival outcomes is required.
Acknowledgements
This work was supported by research grants from the Catholic University of Daegu in 2017.
Notes
No potential conflicts of interest relevant to this article was reported.