Management of diabetic foot ulcers: a narrative review
Article information
Abstract
Diabetic foot ulcers (DFUs) are among the most serious complications of diabetes and are a source of reduced quality of life and financial burden for the people involved. For effective DFU management, an evidence-based treatment strategy that considers the patient's clinical context and wound condition is required. This treatment strategy should include conventional practices (surgical debridement, antibiotics, vascular assessment, offloading, and amputation) coordinated by interdisciplinary DFU experts. In addition, several adjuvant therapies can be considered for nonhealing wounds. In this narrative review, we aim to highlight the current trends in DFU management and review the up-to-date guidelines.
Introduction
The International Working Group on the Diabetic Foot (IWGDF) defines diabetic foot as an infection, ulceration, or destruction of tissues of the foot associated with neuropathy and/or peripheral artery disease in the lower extremity of a person with (a history of) diabetes mellitus [1]. Diabetic foot ulcers (DFUs) are considered one of the most serious complications of diabetes, resulting in reduced quality of life and increased financial burden for the patients involved. In other words, DFU therapy is often challenging, and patients experience financial strain because of the cost of treatment. Therefore, it is essential for patients with diabetes and healthcare professionals to be familiar with the underlying concepts behind the prevention of DFUs. Methods of instruction should be carefully planned to guarantee that patients with diabetes understand, and foot care is offered in accordance with the intended aim. The lifetime risk of a patient with diabetes developing a DFU is 19% to 34%, 50% to 70% of patients with DFUs die within 5 years, and 5% require major amputation [2,3]. Therefore, establishing evidence-based treatment strategies for DFUs is critical. Current therapeutic strategies for DFUs include conventional (surgical debridement, antibiotics, vascular assessment, offloading, and amputation) and adjuvant practices (placental-derived products, sucrose octasulfate-impregnated dressing, leukocyte and platelet-rich fibrin (PRF) patches, hyperbaric oxygen therapy [HBOT], and negative pressure wound therapy [NPWT]) combined with interdisciplinary approaches. In this manuscript, we aim to highlight the current trends in DFU management and review up-to-date guidelines.
Conventional treatment
Since the association between diabetes and foot gangrene was first recognized in the 19th century, DFU treatment has evolved significantly. Initially, DFUs were treated with prolonged bed rest, although it was observed that the wound would reemerge once the patient returned to activity [4]. At the end of the 19th century, Frederick Treves introduced sharp debridement for DFU, followed by the administration of antiseptic cream. In addition, he applied a thick pad of felt plaster to the healed ulcer to reduce pressure and prevent recurrence [4]. Building on this principle, the current treatment strategy for DFU includes local wound care with surgical debridement, dressings promoting a moist wound environment, wound offloading, vascular assessment, active infection control, and glycemic control [5-7].
1. Surgical debridement
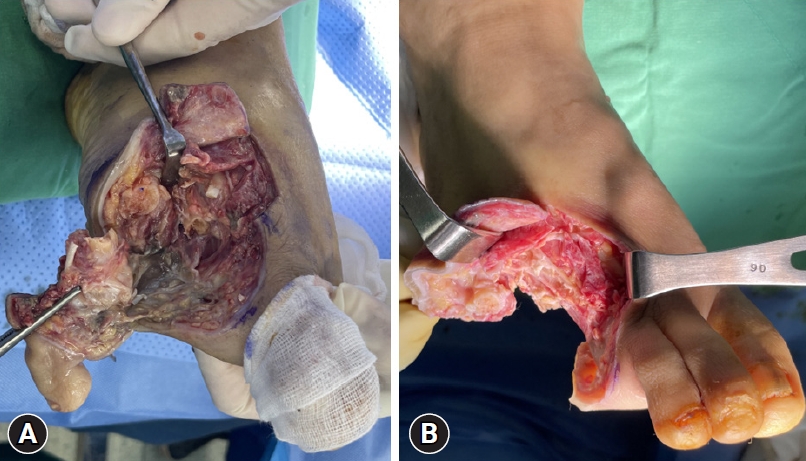
Debridement involves the removal of dead and devitalized tissues from wounds to create a clean wound bed that promotes wound healing (Fig. 1) [6]. This process aids in granulation tissue formation and re-epithelialization and reduces plantar pressures in callused areas. In addition, it is effective in infection control because bacterial proliferation may occur in devitalized tissues, which act as a physical barrier to antibiotic flow and restrict the immune response to infections [8]. Consequently, the IWGDF guidelines recommend sharp debridement as the best standard of care that is preferred over autolytic, biosurgical, hydrosurgical, chemical, or laser debridement [7]. After meticulous debridement, primary wound closure is possible if the soft tissue coverage is adequate and the wound is clean. However, debridement should be repeated every 24 to 72 hours if new necrotic tissue arises and if there is clinical and biochemical evidence of active infection. In addition to debridement, dressings should be selected to control excessive exudates and maintain a moist environment [9].
2. Antibiotics
The choice of antibiotic therapy mainly depends on microbiological findings and antibiotic resistance. Therefore, obtaining deep tissue cultures during debridement is recommended before antibiotic therapy. Swab specimens should be avoided especially in cases of inadequately debrided wounds [6]. In the case of a superficial and stable DFU without evidence of infection, antibiotic therapy is not indicated and antiseptic wound dressings are usually sufficient. In superficial ulcers with mild infections, empiric oral antibiotics targeting Staphylococcus and β-hemolytic streptococci are recommended. In a patient with a deep or potentially limb-threatening infection, initiation of empiric, intravenous, and broad-spectrum antibiotic therapy aimed at common gram-positive and gram-negative bacteria is urgent. A 14-day course of antibiotic therapy is usually sufficient; however, the duration may be longer in cases where bone is involved or the infected tissue has not been removed surgically [10]. Therefore, close clinical and laboratory monitoring is required [7]. Most importantly, adequate acquisition of tissue samples, followed by the timely administration of antibiotics, can potentially maximize tissue rescue and decrease the amputation rate, or at least reduce the size of the amputation [11].
3. Vascular assessment
Peripheral arterial disease (PAD) is known to cause slower DFU healing, increased amputation rates, and higher mortality rates [12]. As adequate blood flow is essential for healing and combating severe infections involving DFUs, appropriate PAD screening and vascular assessment should be performed during DFU care [13]. The IWGDF guidelines suggest that urgent vascular intervention should be considered in patients with one of the following criteria: ankle pressure, <50 mmHg; toe pressure, <30 mmHg; ankle-brachial index, <0.4; or transcutaneous oxygen pressure, <25 mmHg (Fig. 2) [7]. Even with higher pressure levels, patients with extensive tissue loss or infection may benefit from revascularization according to the wound, ischemia, and foot infection classification system [14]. Furthermore, no signs of wound healing within 4 to 6 weeks despite optimal management is an indication for further vascular imaging and revascularization, irrespective of the results of the vascular diagnostic tests. Pharmacological treatments for improving perfusion have not proven beneficial [7]. Instead, emphasis should be placed on reducing the high cardiovascular risk associated with PAD in individuals with diabetes. This includes smoking cessation, management of hypertension and dyslipidemia, and use of antiplatelet drugs [7].
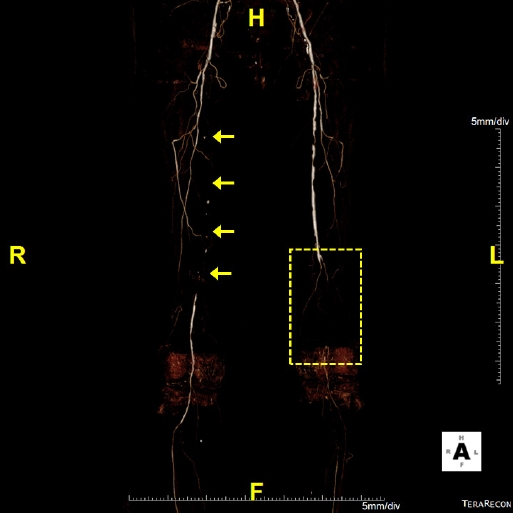
Computed tomography angiography of a patient with ischemic diabetic foot ulcer. The right proximal to middle superficial femoral artery shows total occlusion (arrows) and the ankle-brachial index (ABI) is 0.38. Left distal superficial femoral and popliteal arteries reveal total occlusion and the ABI is not measurable due to poor vascular status (dotted box).
4. Offloading
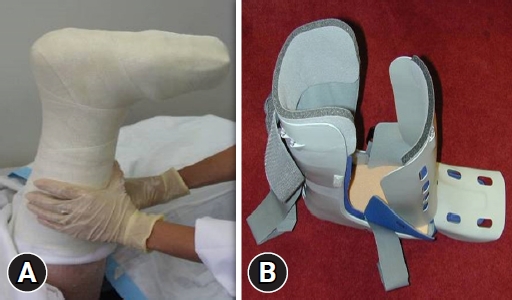
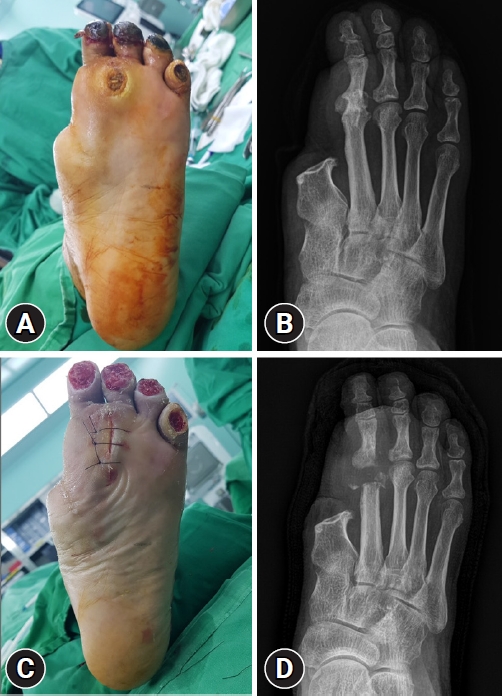
Plantar shear stress and vertical plantar pressure are known causative factors of DFUs [15,16], which result from mechanical loading of the feet during activities and can be aggravated by foot deformities. Therefore, offloading the foot and managing any deformity are essential for preventing and treating DFUs [17]. Offloading can relieve or redistribute plantar pressure to avoid high pressure zones in DFUs and protect the pressure points on the foot. Offloading can be achieved using a variety of devices, including casts, therapeutic shoes, orthoses, felt padding, and foam [18]. Among these, nonremovable knee-high offloading devices such as total contact casts or prefabricated knee-high orthoses are considered first-line recommendations [19]. These are known to reduce peak pressure in the forefoot by up to 87% by redistributing plantar pressure over the entire weight-bearing surface of the foot and are considered more effective than removable devices in terms of time to heal and percentage of wounds healed [20]. If a nonremovable knee-high device is not tolerated, that is, there is significant PAD or infection, a removable knee-high device or ankle-high offloading device can be considered, with a sufficient explanation of the benefit of adherence to wearing the device (Fig. 3). If other forms of biomechanical relief are not available, felted foam can be used, but only in combination with appropriate footwear. If nonsurgical offloading efforts fail to produce satisfactory healing, the IWGDF guidelines recommend surgical approaches such as Achilles tendon lengthening, metatarsal head resection, joint arthroplasty, or metatarsal osteotomy (Fig. 4) [19].
5. Amputation
Despite desperate efforts to rescue the foot, amputation is unavoidable in some cases. Although various types of amputation are possible depending on the extent of DFUs, a careful approach should be taken because limb amputation may increase the patient's physical, economic, and emotional burden [21,22]. The foremost principle to consider when determining the level of amputation is that energy consumption after amputation is inversely proportional to the length of the residual limb [23]. In other words, the more proximal the amputation, the greater the amount of energy that is required during activity. As a result, distal limb-conserving amputations are preferred when possible. In addition, patients should be reminded that positive outcomes can be achieved after amputation because of advances in orthotics, prosthetics, and rehabilitation.
Adjuvant treatment
In addition to conventional treatment modalities, various adjuvant therapies have been studied. Recent IWGDF guidelines indicate that these can be considered for noninfected DFUs that fail to heal after 4 to 6 weeks of optimal management. However, there is insufficient evidence to support the use of biologically active products (collagen, growth factors, and bioengineered tissue), topical antiseptics, and antimicrobial dressings for routine DFU management.
1. Placental-derived products
Placental-derived products include dehydrated amnion-chorion grafts, dehydrated human amniotic membranes, cryopreserved placental membranes, and dehydrated human umbilical cords. They contain cytokine growth factors, collagen, and other extracellular matrix components that promote tissue regeneration. Furthermore, the application of placental-derived products enhances dermal fibroblast proliferation and recruits mesenchymal stem cells to the injury site [24]. Multiple studies have suggested that the use of placental-derived products can improve DFU healing and the time to heal, which supports their effectiveness as adjuvant treatments [25-27].
2. Sucrose octasulfate-impregnated dressing
DFUs have a prolonged inflammatory phase with fibroblast dysfunction, impaired neovascularization, and increased matrix metalloproteinase levels, which delay wound healing through the degradation of growth factors and destruction of the extracellular matrix [28,29]. The potassium salt of sucrose octasulfate inhibits excess matrix metalloproteinases, and its unique structure can interact with growth factors, which may eventually stimulate tissue formation [30].
A double-blind multinational randomized controlled trial (RCT) indicated that the use of a sucrose octasulfate dressing improved the rate of wound closure over 20 weeks in patients with neuroischemic DFUs compared with the use of a control dressing [31]. Therefore, in neuroischemic foot ulcers, where the change in ulcer area has been inadequate with the best standard of care, there is sufficient evidence to consider the use of sucrose octasulfate-impregnated dressings.
3. Leukocyte and platelet-rich fibrin patch
Platelet-rich plasma or PRF may promote DFU healing by releasing cytokines and growth factors involved in tissue repair, angiogenesis, and inflammation. Recently developed multilayered patches composed of autologous leukocytes, platelets, and fibrin can be prepared at the bedside without additional reagents [32]. A high-quality multicenter double-blind RCT demonstrated that patients with hard-to-heal DFUs who were treated with standard care and multilayered patches showed significant improvements in wound healing, time to healing, and wound area reduction. Based on these results, the IWGDF guidelines recommend the use of autologous leukocyte and PRF patches in case standard care alone is ineffective [33].
4. Hyperbaric oxygen therapy
In HBOT, the patients inhale 100% oxygen at greater than 1 atmosphere of pressure, which has been recognized to promote local tissue oxygenation, improve tissue hypoxia, and reduce wound infection through an antibacterial effect [34]. Despite its long history as a treatment for DFUs, the efficacy of HBOT remains controversial. Some systematic reviews concluded that HBOT is advantageous in promoting healing, minimizing the size of DFUs, and reducing the amputation rate, whereas others found no significant effect of HBOT on nonischemic DFUs [35]. Although routinely applying HBOT to all patients with DFUs is not recommended, it may play a role in promoting ulcer healing and reducing the amputation rate in patients with ischemic DFUs (Fig. 5). In this context, the IWGDF guidelines suggest the use of HBOT as an adjunct therapy for neuroischemic or ischemic DFUs when the standard of care alone fails [7].
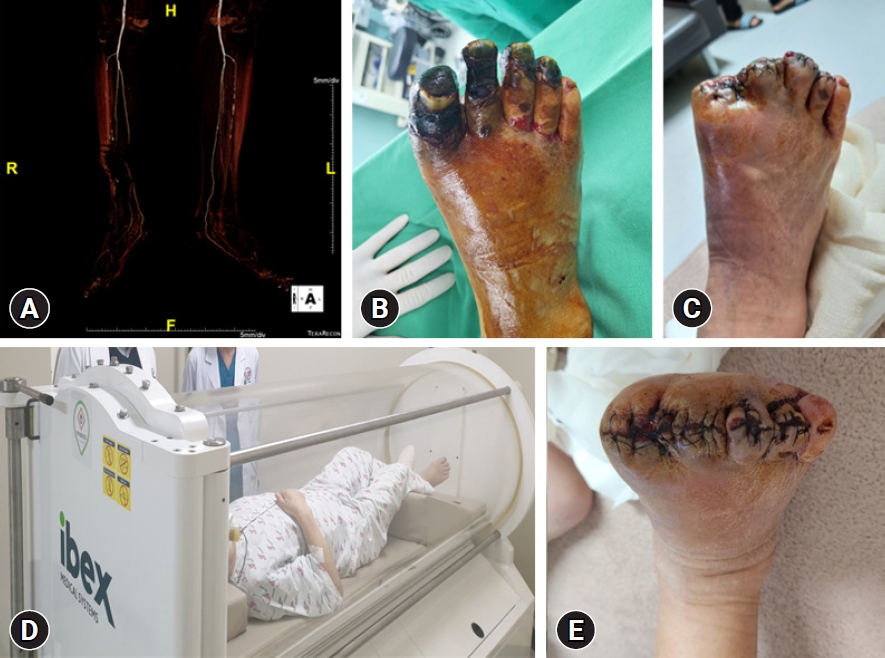
A 73-year-old male with a 3-month history of diabetic foot ulcer. (A) Computed tomography angiography shows multifocal mild to moderate stenosis at both the superficial femoral artery and popliteal arteries. (B) Ischemic necrosis accompanied by infectious edema is detected on the first to fourth toes. (C) Toe amputation has been performed. (D) The patient has undergone adjuvant hyperbaric therapy both preoperatively for wound demarcation and postoperatively for wound healing. (E) The amputation wound has healed with improved infection and swelling.
5. Negative pressure wound therapy
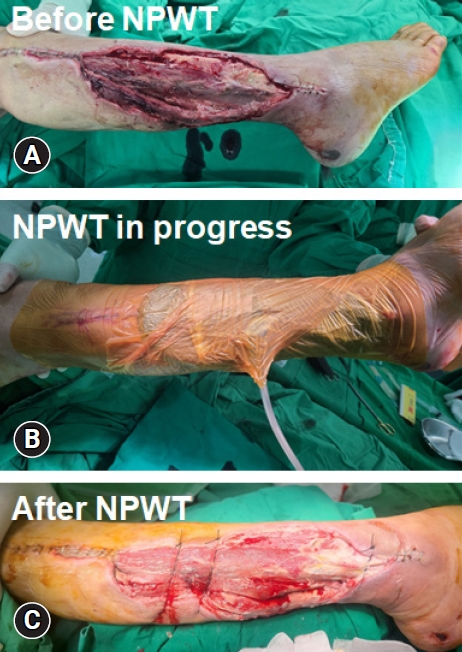
NPWT is commonly employed in wound management because of the ability to collect substantial amounts of wound fluid using a vacuum device (Fig. 6). This feature reduces the frequency of dressing changes, helps maintain cleanliness in challenging anatomical wound areas, and diminishes odors. Additionally, it is believed that the application of vacuum forces through NPWT contributes to wound healing by enhancing blood flow, removing infectious material, and facilitating the approximation of the wound edges. A recent systematic review that examined 11 RCTs comparing NPWT with standard dressing changes demonstrated that NPWT resulted in a higher rate of complete healing, shorter healing time, and fewer instances of amputation. No notable differences were observed in the occurrence of treatment-related adverse effects [9].
Interdisciplinary approach
In addition to the aforementioned efforts, an interdisciplinary approach is becoming the mainstay of treatment. Because DFU is considered a sign of multi-organ disease, an interdisciplinary approach is needed to effectively treat and prevent it [36]. An interdisciplinary DFU team should include a group of healthcare professionals with wide knowledge of different aspects of diabetic foot care [37]. The IWGDF guidelines suggest at least three levels of foot care management with interdisciplinary specialists (Table 1) [7]. If it is not possible to form a full team, an effort should be made to establish one step-by-step, including as many disciplines as possible. Together with mutual respect and understanding, these professionals try to focus on the treatment of existing DFUs, secondary prevention, and prevention of recurrence. In other words, the team is designed to cope with the needs of patients requiring chronic care instead of responding to acute problems [7]. As a result, numerous studies and systematic reviews have shown the positive effects of interdisciplinary care in reducing wound healing times, amputation rates, and the severity of amputation [38-40].
Conclusion
Despite well-established guidelines, DFU treatment has not achieved satisfactory clinical outcomes. To manage this complex, fastidious disease, a clear clinical judgment considering the patient's clinical context and wound condition should be made following an evidence-based treatment strategy. In addition, an interdisciplinary approach can effectively aid treatment and prevention. Although the prevalence of diabetes is increasing in underdeveloped countries, there is still a lack of knowledge and education regarding this disease. Although the principles underlying the standard of care are sound, a notable disparity exists between the wound healing outcomes that we currently achieve and those that we aspire to attain. The provision of diabetes-related preventive care could potentially be improved by increasing accessibility to diabetes education.
Notes
Ethical statements
Written informed consent was obtained for publication of this study and accompanying images.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study was supported by the Hallym University Research Fund.
Author contributions
Conceptualization: JK, CYA, YCC, JC; Data curation: JK, YCC; Formal analysis: ON, JC; Funding acquisition, Supervision: JC; Investigation: JK, CYA; Resources: CYA; Visualization, Software: YCC; Writing-original draft: JK, JC; Writing-review & editing: ON.