PubMed Central, CAS, DOAJ, KCI

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Volume 40(4); 2023 > Article
-
Original article
Cortical thickness of the rostral anterior cingulate gyrus is associated with frailty in patients with end-stage renal disease undergoing hemodialysis in Korea: a cross-sectional study -
Sang Hyun Jung1,*
 , Jong Soo Oh2,*
, Jong Soo Oh2,* , So-Young Lee1
, So-Young Lee1 , Hye Yun Jeong1
, Hye Yun Jeong1
-
Journal of Yeungnam Medical Science 2023;40(4):381-387.
DOI: https://doi.org/10.12701/jyms.2022.00941
Published online: March 24, 2023
1Division of Nephrology, Department of Internal Medicine, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, Korea
2Department of Psychiatry, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, Korea
- Corresponding author: Hye Yun Jeong, MD Division of Nephrology, Department of Internal Medicine, CHA Bundang Medical Center, CHA University School of Medicine, 59 Yatap-ro, Bundang-gu, Seongnam 13496, Korea Tel: +82-31-780-5953 • Fax: +82-31-780-5219 • E-mail: heunj85@chamc.co.kr
- *These authors contributed equally to this work.
Copyright © 2023 Yeungnam University College of Medicine, Yeungnam University Institute of Medical Science
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,232 Views
- 39 Download
Abstract
-
Background
- Frailty is defined as a condition of being weak and delicate, and it represents a state of high vulnerability to adverse health outcomes. Recent studies have suggested that the cingulate gyrus is associated with frailty in the elderly population. However, few imaging studies have explored the relationship between frailty and the cingulate gyrus in patients with end-stage renal disease (ESRD) undergoing hemodialysis.
-
Methods
- Eighteen right-handed patients with ESRD undergoing hemodialysis were enrolled in the study. We used the FreeSurfer software package to estimate the cortical thickness of the regions of interest, including the rostral anterior, caudal anterior, isthmus, and posterior cingulate gyri. The Beck Depression Inventory, Beck Anxiety Inventory, and laboratory tests were also conducted.
-
Results
- The cortical thickness of the right rostral anterior cingulate gyrus (ACG) was significantly correlated with the Fried frailty index, age, and creatinine level. Multiple regression analysis indicated that the cortical thickness of the right rostral ACG was associated with frailty after controlling for age and creatinine level.
-
Conclusion
- Our results indicate that the cortical thickness of the rostral ACG may be associated with frailty in patients with ESRD on hemodialysis and that the rostral ACG may play a role in the frailty mechanism of this population.
- Frailty is primarily defined as a condition of being weak and delicate, which is correlated with chronologic age [1]. It is well known that individuals who are frail are vulnerable to adverse health outcomes [2,3]. Chronic kidney disease (CKD), which involves the progressive loss of kidney function over months or years resulting in reduced glomerular filtration rate, has important phenotypic similarities with frailty that reflect premature aging, such as vascular disease, muscle wasting, and osteoporosis [4]. Indeed, the prevalence of frailty has been reported to be 26% to 68% in patients aged >20 years undergoing dialysis [1,5,6], whereas it was only 7% in patients aged ≥65 years who were community-dwelling [7].
- Many studies have shown that psychiatric problems such as cognitive function impairment, memory loss, and depression are associated with frailty [8-10]. Although patients with end-stage renal disease (ESRD) have a much higher risk of psychiatric complications, studies investigating the relationship between brain changes and frailty in patients with CKD are scarce. Moreover, few imaging studies have explored the association between frailty and brain structure, reflecting a change in the psychiatric state of these patients. A recent study showed that patients with ESRD exhibited significantly decreased functional connectivity within the fronto-cerebellar circuits, including the cingulate cortex [11]. In addition, a voxel-based morphometry study showed structural changes in the cingulate cortex of patients with ESRD [12].
- The cingulate cortex is located within the medial cerebral cortex. It is an important component of the limbic system and is involved in emotion formation and processing [13], learning, and memory [14]. The combination of these three functions makes the cingulate gyri closely linked to behavioral outcomes related to motivation [15]. In addition, the cingulate cortex is connected to the motor cortex, which is implicated in motor control [16]. As frailty can be associated with emotion, cognition, motivation, and motor control [9], it is possible that the cingulate plays an important role in regulating frailty in patients with CKD.
- In this study, we aimed to investigate the relationship between frailty and structural changes in the cingulate cortex of patients with ESRD undergoing hemodialysis treatment.
Introduction
- Ethical statements: All study procedures were approved by the Institutional Review Board (IRB) of CHA Bundang Medical Center (IRB No: BD2015-07117-005). The study was conducted in accordance with the Declaration of Helsinki and the principles of Good Clinical Practice. Written informed consent was obtained from all patients.
- 1. Subjects
- The study included data from the Artificial Kidney Unit of CHA Bundang Medical Center. Eighteen volunteers with ESRD undergoing maintenance hemodialysis were recruited for this study. All the participants were Korean and right-handed. The inclusion criteria were age of >18 years and treatment with hemodialysis three times per week (≥12 hours/week) for at least 3 months without renal transplantation. The exclusion criteria included the occurrence of any brain lesions, tumors, or stroke according to medical history, and any current diagnosis or lifetime history of major neurocognitive disorders, schizophrenia, mood disorders (including major depressive disorder and bipolar disorder), anxiety disorders, alcohol and substance abuse or dependence, intellectual disability, other serious medical or neurological disorders, pregnancy, and contraindications for brain magnetic resonance (MR) scanning, including metal implants.
- 2. Definition of frailty
- We adopted the Fried criteria as the definition of frailty [17]. Frailty was measured as a phenotype based on five components: (1) shrinking (self-reported unintentional weight loss of >10 pounds in the past year based on dry weight, i.e., the weight of an individual undergoing hemodialysis without the excess fluid that accumulates between dialysis treatments, which is more representative of his/her weight in the context of normal kidney function), (2) weakness (grip strength below an established cutoff based on sex), (3) exhaustion (self-reported), (4) low activity (kcal/week below an established cutoff), and (5) slow walking speed (time taken to walk 4 m above an established cutoff according to sex) [17]. A score of one was assigned to each measured component. The aggregate frailty score was calculated as the sum of the component scores (range, 0–5) [1].
- 3. Clinical variables
- Patient demographic and clinical data, including age, sex, etiology of ESRD (e.g., diabetes mellitus and hypertension), mean blood pressure, and body mass index, were obtained by medical record review. Laboratory data were collected at the time of patient enrollment, including Kt/V, serum white blood cell counts, and levels of 25-hydroxy vitamin D, uric acid, serum hemoglobin, protein, albumin, blood urea nitrogen, creatinine, calcium, phosphate, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, C-reactive protein, glucose, and hemoglobin A1C.
- 4. Psychiatric clinical severity
- The patients with ESRD were assessed at baseline for clinical severity using the Beck Depression Inventory (BDI) and Beck Anxiety Inventory (BAI). The BDI is a commonly used instrument for quantifying depression. It is a 21-question self-reported inventory used to assess the type and degree of depression based on the symptoms experienced by the patient [18]. The questionnaire consists of questions about emotional, cognitive, motivational, physiological, and other symptoms that reflect how the participants felt over the past week.
- The BAI is a commonly used 21-question multiple-choice self-reported inventory used to measure the severity of anxiety in children and adults. The questions used in this measure pertain to any symptoms of anxiety that the subject experienced during the week prior to testing [19].
- 5. Magnetic resonance imaging acquisition and data processing
- All participants underwent MR imaging on the same 3.0T GE Signa HDxt scanner (GE Healthcare, Milwaukee, WI, USA) equipped with an eight-channel phased array head coil. The parameters for three-dimensional T1-weighted fast spoiled gradient recalled echo (T1-FSPGR) image acquisition were as follows: repetition time, 6.3 milliseconds; echo time, 2.1 milliseconds; flip angle, 12°; slice thickness, 1 mm; field of view, 25.6 cm; 256×256 matrix; and isotropic voxel size, 1×1×1 mm3.
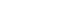
- We used the FreeSurfer ver. 5.3 software package to create a three-dimensional model of the cortical surface to estimate the cortical thickness of the regions of interest (ROIs), including the rostral anterior, caudal anterior, isthmus, and posterior cingulate gyri. We extracted the results of cortical parcellation using the Desikan-Killiany atlas. The postprocessing outputs for each subject were visually examined to ensure processing accuracy and image quality.
- 6. Statistical analysis
- The mean thickness was extracted for each participant, and the table file was analyzed using IBM SPSS ver. 23 (IBM Corp., Armonk, NY, USA). Partial correlation analysis was used for the relationship between the cortical thickness of the cingulate cortex ROIs and frailty, controlling for the demographic findings, psychological measures, and medical laboratory results, all of which could influence frailty. A multiple regression analysis was performed to confirm this relationship. To visualize the results, we used the following method proposed by Hagler et al. [20]. Briefly, operating within the framework of the FreeSurfer software package, a surface-based version of the cluster size exclusion method was used for multiple comparison correction. This method generates ROIs on the cortical surface using a sliding threshold of cluster exclusion followed by cluster growth.
Methods
- 1. Demographic and clinical characteristics of the subjects
- The demographic and clinical characteristics of the patients with ESRD undergoing hemodialysis are shown in Table 1. Among the demographic and clinical variables, only age (r=0.574, p=0.013) and creatinine level (r=–0.529, p=0.024) were significantly correlated with the frailty scores (Table 2).
- 2. Cortical thickness of the rostral anterior cingulate gyrus was significantly correlated with frailty in patients with end-stage renal disease undergoing hemodialysis
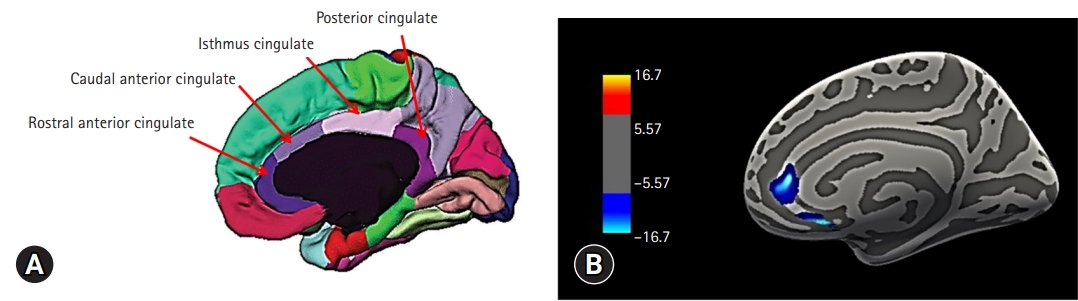
- We found that the cortical thickness of the right rostral anterior cingulate gyrus (ACG) (r=–0.532, p=0.023) was significantly correlated with frailty in patients with ESRD. When we used a voxel-wise correlational analysis with the FreeSurfer program to confirm this finding, it also revealed that the cortical thickness values of the right rostral ACG, among the cingulate gyrus ROIs, were negatively correlated with the frailty scores in patients with ESRD (Fig. 1).
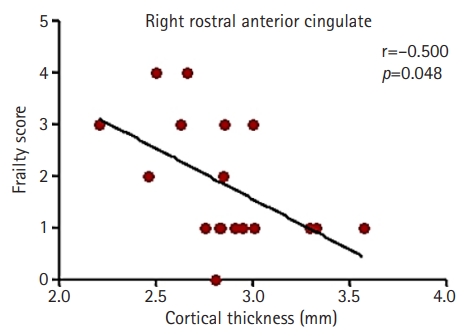
- Partial correlation analysis also showed a significant correlation between the cortical thickness of the right rostral ACG and Fried scores of frailty after controlling for age and creatinine levels (r=–0.500, p=0.048) (Fig. 2). Multiple regression analysis also showed that the cortical thickness of the right rostral ACG was significantly associated with the frailty scores (Table 3).
Results
- To our knowledge, this study is the first to demonstrate that the cortical thickness values of the right rostral ACG are significantly negatively correlated with frailty in patients with ESRD. In addition, this significant correlation remained after controlling for age and creatinine levels. This result suggests that cortical thickness reduction in the ACG is independently associated with frailty in patients with ESRD.
- In previous studies that investigated the factors associated with frailty, a significant association between frailty and several sociodemographic, physical, biological, psychological, and lifestyle factors was found. Several authors have suggested that the development of frailty typically increases with age, revealing a significantly positive association between age and frailty. Furthermore, frailty is more common in patients who are elderly with CKD than in the general population, possibly because frailty is associated with protein-energy wasting, sarcopenia, and several other complications of CKD [21]. In this study, we also showed a positive relationship between age and frailty scores in patients with ESRD.
- It is well known that malnutrition and frailty are closely associated [22] and share common pathophysiological pathways. Although the etiologies of these conditions differ, loss of body tissue results in wasting and chronic inflammation, which are common phenotypes of both conditions [23]. Blood protein markers such as albumin and prealbumin have been previously considered biomarkers of malnutrition [24]. However, these are no longer recommended as markers of malnutrition because they are affected not only by nutrition but also by many other risk factors such as infection, inflammation, liver function, and fluid status [25]. Recently, Zhang et al. [24] suggested that hemoglobin and total cholesterol could be useful markers of malnutrition in those who are elderly, whereas Canaud et al. [26] showed that serum creatinine levels could be used as a nutritional and muscle mass marker in patients with ESRD. In the present study on patients with ESRD, we also failed to show a significant relationship between albumin level and frailty, although there was a statistically significant association between frailty score and creatinine level.
- Several studies have shown that cognitive impairment [8] and depression [10] are associated with frailty. Among the five frailty phenotypes, performance functions, including weakness and slowness, are known to affect cognitive function and depression [27]. A previous study on women who are elderly suggested that depressive symptoms are strongly associated with physical performance [28], and Kang et al. [29] showed that low grip strength and slow walking speed were associated with decreased cognition in women who are elderly. A recent systematic review also indicated that cognitive function is related to frailty in old age, and slowness and muscle weakness are particularly associated with cognition [30]. It is generally accepted that CKD is associated with cognitive impairment [31] and depression [32,33]. In addition, previous studies have shown low performance test scores in patients with ESRD [31]. Given these findings, deterioration of physical performance in patients with ESRD may be associated with decreased cognitive function and depression. Since we excluded patients with neurocognitive disorders, we did not find a relationship between cognitive function and brain structure in patients with ESRD.
- The cingulate region of the brain is well known to play an important role in the regulation of emotion [34,35]. In addition, the attentive function of the cingulate region may be required for the emotional regulation performed by the temporal lobe structures, including the amygdala. Previous studies have indicated that the anterior cingulate region is closely linked to motivation and motor function [16,36]. Overall, the anterior cingulate seems to be linked to various functions related to frailty and may have significant effects on frailty. According to previous studies, there is a close relationship between physical frailty and decreased capacity in specific brain regions, including the cerebellum, hippocampus, frontal gyrus, and anterior cingulate [37]. In particular, the anterior cingulate is associated with low activity. Furthermore, a structural and functional study showed that fronto-cerebellar circuits, including the anterior cingulate, are altered in patients with ESRD [11]. These results suggest that the anterior cingulate, in addition to other brain regions, is related to frailty in patients with ESRD. The results of the present study are also consistent with those of earlier studies in which the anterior cingulate was implicated in frailty. In this study, we also showed that the right rostral ACG was significantly negatively correlated with frailty scores in patients with ESRD.
- However, the mechanism by which ESRD affects the brain and causes frailty remains unclear. A previous brain autopsy study showed that Alzheimer’s disease pathology and macroinfarcts contribute to progressive physical frailty in old age [38]. It is known that there is a high incidence of dementia [39] and cerebral infarction [40] in patients with ESRD. We speculate that ESRD affects the brain in a manner similar to Alzheimer's pathology and macroinfarcts. It is likely that the cingulate gyri are sensitive to this type of brain deterioration.
- The strength of our study is that we demonstrated, for the first time, the association between cortical thickness and frailty in patients with ESRD. In addition, our study had the advantage of calculating the frailty score based on the direct performance of a physical function test.
- This study had several limitations. First, the sample size is small. Second, this study was conducted on patients who visited the hospital without completely controlling for several biases, including age-related selection bias. Third, this was a cross-sectional study that did not reveal the relationship between cause and effect. Fourth, medications, such as antihypertensive or antidiabetic drugs, may have affected the cortical thickness changes in the subjects. Finally, as this was a prospective observational cohort study, we could not perform an analysis to compare the results to similar age groups with normal renal function, or to compare patients with different renal functions.
- In conclusion, this study suggests that the rostral ACG plays a role in the frailty mechanism in patients with ESRD undergoing hemodialysis. Further research involving a larger number of patients and longitudinal assessments are warranted in the future.
Discussion
-
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
-
Funding
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C2750).
-
Author contributions
Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Investigation, Resources: all authors; Visualization, Software: SHJ, JSO; Supervision: SHJ, SYL, HYJ; Validation: SHJ, SYL; Writing-original draft: SHJ, JSO; Writing-review & editing: all authors.
Notes
Values are presented as number only, mean±standard deviation, number (%), or median (interquartile range).
HD, hemodialysis; BDI, Beck Depression Inventory; BAI, Beck Anxiety Inventory; MBP, mean blood pressure; 25-OH vitamin D, 25-hydroxy vitamin D; WBC, white blood cell; BUN, blood urea nitrogen; HDL, high-density lipoprotein; LDL, low-density lipoprotein; CRP, C-reactive protein; HgbA1c, glycated hemoglobin; Kt/V, dialyzer clearance of urea×dialysis time/volume of urea distribution.
| Variable | r | p-value |
|---|---|---|
| Age (yr) | 0.574 | 0.013a) |
| HD duration (mo) | 0.302 | 0.223 |
| Intracranial volume (mL) | –0.253 | 0.311 |
| Body mass index (kg/m2) | –0.198 | 0.432 |
| BDI | 0.515 | 0.060 |
| BAI | 0.439 | 0.116 |
| MBP (mmHg) | –0.222 | 0.391 |
| 25-OH vitamin D (ng/dL) | –0.239 | 0.373 |
| Uric acid (mg/dL) | –0.184 | 0.465 |
| Hemoglobin (g/dL) | –0.059 | 0.815 |
| WBC (/µL) | –0.102 | 0.687 |
| Protein (g/dL) | –0.100 | 0.694 |
| Albumin (mg/dL) | –0.374 | 0.126 |
| BUN (mg/dL) | –0.232 | 0.354 |
| Creatinine (mg/dL) | –0.529 | 0.024a) |
| Calcium (mg/dL) | –0.320 | 0.196 |
| Phosphate (mg/dL) | –0.088 | 0.729 |
| Total cholesterol (µg/dL) | 0.171 | 0.499 |
| HDL cholesterol (mg/dL) | –0.204 | 0.416 |
| LDL cholesterol (mg/dL) | 0.127 | 0.617 |
| CRP (mg/dL) | 0.242 | 0.334 |
| Glucose (mg/dL) | 0.057 | 0.823 |
| HgbA1c (%) | 0.472 | 0.121 |
| Kt/V | –0.553 | 0.097 |
HD, hemodialysis; BDI, Beck Depression Inventory; BAI, Beck Anxiety Inventory; MBP, mean blood pressure; 25-OH vitamin D, 25-hydroxy vitamin D; WBC, white blood cell; BUN, blood urea nitrogen; HDL, high-density lipoprotein; LDL, low-density lipoprotein; CRP, C-reactive protein; HgbA1c, glycated hemoglobin; Kt/V, dialyzer clearance of urea×dialysis time/volume of urea distribution.
a) p<0.05.
| Variable | Standard β | t | p-value |
|---|---|---|---|
| Creatinine | –0.255 | –1.179 | 0.258 |
| Age | 0.362 | 1.711 | 0.109 |
| Right rostral anterior cingulate | –0.392 | –2.162 | 0.048 |
- 1. Lee SY, Yang DH, Hwang E, Kang SH, Park SH, Kim TW, et al. The prevalence, association, and clinical outcomes of frailty in maintenance dialysis patients. J Ren Nutr 2017;27:106–12.ArticlePubMed
- 2. Painter P, Roshanravan B. The association of physical activity and physical function with clinical outcomes in adults with chronic kidney disease. Curr Opin Nephrol Hypertens 2013;22:615–23.ArticlePubMed
- 3. Roshanravan B, Khatri M, Robinson-Cohen C, Levin G, Patel KV, de Boer IH, et al. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis 2012;60:912–21.ArticlePubMedPMC
- 4. Kooman JP, Kotanko P, Schols AM, Shiels PG, Stenvinkel P. Chronic kidney disease and premature ageing. Nat Rev Nephrol 2014;10:732–42.ArticlePubMedPDF
- 5. Johansen KL, Chertow GM, Jin C, Kutner NG. Significance of frailty among dialysis patients. J Am Soc Nephrol 2007;18:2960–7.ArticlePubMed
- 6. McAdams-DeMarco MA, Suresh S, Law A, Salter ML, Gimenez LF, Jaar BG, et al. Frailty and falls among adult patients undergoing chronic hemodialysis: a prospective cohort study. BMC Nephrol 2013;14:224.ArticlePubMedPMCPDF
- 7. Wilhelm-Leen ER, Hall YN, K Tamura M, Chertow GM. Frailty and chronic kidney disease: the Third National Health and Nutrition Evaluation Survey. Am J Med 2009;122:664–71.ArticlePubMedPMC
- 8. Jacobs JM, Cohen A, Ein-Mor E, Maaravi Y, Stessman J. Frailty, cognitive impairment and mortality among the oldest old. J Nutr Health Aging 2011;15:678–82.ArticlePubMedPDF
- 9. Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 2004;59:255–63.ArticlePubMed
- 10. Ní Mhaoláin AM, Fan CW, Romero-Ortuno R, Cogan L, Cunningham C, Kenny RA, et al. Frailty, depression, and anxiety in later life. Int Psychogeriatr 2012;24:1265–74.ArticlePubMed
- 11. Qiu Y, Lv X, Su H, Jiang G, Li C, Tian J. Structural and functional brain alterations in end stage renal disease patients on routine hemodialysis: a voxel-based morphometry and resting state functional connectivity study. PLoS One 2014;9:e98346.ArticlePubMedPMC
- 12. Papoiu AD, Emerson NM, Patel TS, Kraft RA, Valdes-Rodriguez R, Nattkemper LA, et al. Voxel-based morphometry and arterial spin labeling fMRI reveal neuropathic and neuroplastic features of brain processing of itch in end-stage renal disease. J Neurophysiol 2014;112:1729–38.ArticlePubMedPMC
- 13. Hadland KA, Rushworth MF, Gaffan D, Passingham RE. The effect of cingulate lesions on social behaviour and emotion. Neuropsychologia 2003;41:919–31.ArticlePubMed
- 14. Sutherland RJ, Whishaw IQ, Kolb B. Contributions of cingulate cortex to two forms of spatial learning and memory. J Neurosci 1988;8:1863–72.ArticlePubMedPMC
- 15. Hayden BY, Platt ML. Neurons in anterior cingulate cortex multiplex information about reward and action. J Neurosci 2010;30:3339–46.ArticlePubMedPMC
- 16. Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci 2001;2:417–24.ArticlePubMedPDF
- 17. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–56.ArticlePubMed
- 18. Wang YP, Gorenstein C. Assessment of depression in medical patients: a systematic review of the utility of the Beck Depression Inventory-II. Clinics (Sao Paulo) 2013;68:1274–87.ArticlePubMedPMC
- 19. Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 1988;56:893–7.ArticlePubMed
- 20. Hagler DJ Jr, Saygin AP, Sereno MI. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage 2006;33:1093–103.ArticlePubMed
- 21. Kim JC, Kalantar-Zadeh K, Kopple JD. Frailty and protein-energy wasting in elderly patients with end stage kidney disease. J Am Soc Nephrol 2013;24:337–51.ArticlePubMed
- 22. Wei K, Nyunt MS, Gao Q, Wee SL, Yap KB, Ng TP. Association of frailty and malnutrition with long-term functional and mortality outcomes among community-dwelling older adults: results from the Singapore Longitudinal Aging Study 1. JAMA Netw Open 2018;1:e180650.ArticlePubMedPMC
- 23. Wei K, Nyunt MS, Gao Q, Wee SL, Ng TP. Frailty and malnutrition: related and distinct syndrome prevalence and association among community-dwelling older adults: Singapore Longitudinal Ageing Studies. J Am Med Dir Assoc 2017;18:1019–28.ArticlePubMed
- 24. Zhang Z, Pereira SL, Luo M, Matheson EM. Evaluation of blood biomarkers associated with risk of malnutrition in older adults: a systematic review and meta-analysis. Nutrients 2017;9:829.ArticlePubMedPMC
- 25. Omran ML, Morley JE. Assessment of protein energy malnutrition in older persons, part II: laboratory evaluation. Nutrition 2000;16:131–40.ArticlePubMed
- 26. Canaud B, Granger Vallée A, Molinari N, Chenine L, Leray-Moragues H, Rodriguez A, et al. Creatinine index as a surrogate of lean body mass derived from urea Kt/V, pre-dialysis serum levels and anthropometric characteristics of haemodialysis patients. PLoS One 2014;9:e93286.ArticlePubMedPMC
- 27. Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment: a review of the evidence and causal mechanisms. Ageing Res Rev 2013;12:840–51.ArticlePubMed
- 28. Lee YC. A study of the relationship between depression symptom and physical performance in elderly women. J Exerc Rehabil 2015;11:367–71.ArticlePubMedPMCPDF
- 29. Kang JY, Kim CH, Sung EJ, Shin HC, Shin WJ, Jung KH. The association between frailty and cognition in elderly women. Korean J Fam Med 2016;37:164–70.ArticlePubMedPMC
- 30. Brigola AG, Rossetti ES, Dos Santos BR, Neri AL, Zazzetta MS, Inouye K, et al. Relationship between cognition and frailty in elderly: a systematic review. Dement Neuropsychol 2015;9:110–9.ArticlePubMedPMC
- 31. Kurella M, Chertow GM, Luan J, Yaffe K. Cognitive impairment in chronic kidney disease. J Am Geriatr Soc 2004;52:1863–9.ArticlePubMed
- 32. Cukor D, Peterson RA, Cohen SD, Kimmel PL. Depression in end-stage renal disease hemodialysis patients. Nat Clin Pract Nephrol 2006;2:678–87.ArticlePubMedPDF
- 33. Kimmel PL, Peterson RA. Depression in end-stage renal disease patients treated with hemodialysis: tools, correlates, outcomes, and needs. Semin Dial 2005;18:91–7.ArticlePubMed
- 34. Maddock RJ, Garrett AS, Buonocore MH. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum Brain Mapp 2003;18:30–41.ArticlePubMed
- 35. Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport 1997;8:1057–61.ArticlePubMed
- 36. Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain 1995;118(Pt 1):279–306.ArticlePubMed
- 37. Chen WT, Chou KH, Liu LK, Lee PL, Lee WJ, Chen LK, et al. Reduced cerebellar gray matter is a neural signature of physical frailty. Hum Brain Mapp 2015;36:3666–76.ArticlePubMedPMC
- 38. Buchman AS, Yu L, Wilson RS, Schneider JA, Bennett DA. Association of brain pathology with the progression of frailty in older adults. Neurology 2013;80:2055–61.ArticlePubMedPMC
- 39. Murray AM, Tupper DE, Knopman DS, Gilbertson DT, Pederson SL, Li S, et al. Cognitive impairment in hemodialysis patients is common. Neurology 2006;67:216–23.ArticlePubMed
- 40. Cherng YG, Lin CS, Shih CC, Hsu YH, Yeh CC, Hu CJ, et al. Stroke risk and outcomes in patients with chronic kidney disease or end-stage renal disease: two nationwide studies. PLoS One 2018;13:e0191155.ArticlePubMedPMC
References
Figure & Data
References
Citations


 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine PubReader
PubReader ePub Link
ePub Link Cite
Cite