PubMed Central, CAS, DOAJ, KCI

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Volume 40(4); 2023 > Article
-
Original article
Long-term supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine, and glutamine for pressure ulcer in sedentary older adults: a retrospective matched case-control study -
Igor Kisil1,*
 , Yuri Gimelfarb2,*
, Yuri Gimelfarb2,*
-
Journal of Yeungnam Medical Science 2023;40(4):364-372.
DOI: https://doi.org/10.12701/jyms.2022.00899
Published online: February 17, 2023
1Medical – Care Hospital, Bat Yam, Israel
2AMHC, affiliated to the Sackler Faculty of Medicine, Tel Aviv University, Bat Yam, Israel
- Corresponding author: Igor Kisil, MD, PhD Department of Chronic Mechanical Ventilation, Medical – Care Hospital, 67 Haatzmaut Blvd., Bat Yam, Israel Tel: +972-3-500-8897 • E-mail: igork958@gmail.com
- *These authors contributed equally to this work.
•This study was presented at the 24th Annual Congress of Israel Gerontological Association (2022, July), Tel Aviv, Israel.
Copyright © 2023 Yeungnam University College of Medicine, Yeungnam University Institute of Medical Science
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,213 Views
- 139 Download
- 1 Crossref
Abstract
-
Background
- Growing evidence suggests that beta-hydroxy-beta-methylbutyrate (HMB), arginine (Arg), and glutamine (Gln) positively affect wound recovery. This study investigated the effects of long-term administration of HMB/Arg/Gln on pressure ulcer (PU) healing in sedentary older adults admitted to geriatric and rehabilitation care facilities.
-
Methods
- This was a pilot retrospective case (standard of care and HMB/Arg/Gln)-control (standard of care alone) clinical study. Outcome measures were relative healing rates and Pressure Ulcer Scale for Healing (PUSH) scores (calculated after 4, 8, 12, 16, and 20 weeks) and time to healing.
-
Results
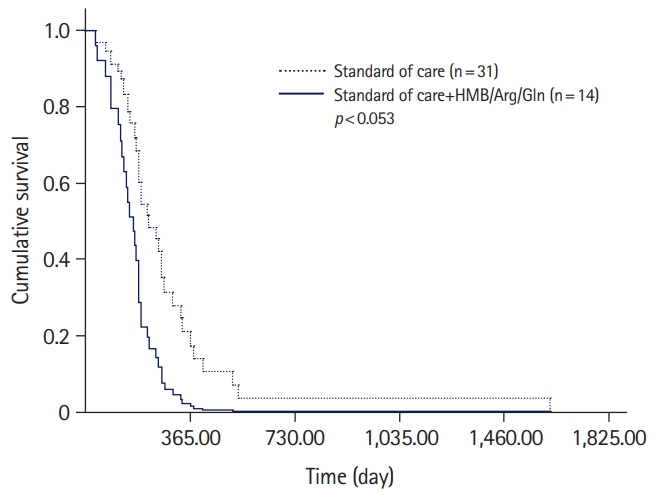
- The study subpopulation was comprised of 14 participants (four males, 28.6%) with the median age of 85.5 years (interquartile range [IQR], 82.0–90.2 years). The control subpopulation was comprised of 31 participants (18 males, 58.1%) with the median age of 84.0 years (IQR, 78.0–90.0 years). At the beginning of follow-up, there were no statistically significant demographic (sex and age) and clinical (main diagnosis, baseline area, and PU perimeter) differences between the groups. During the study period, there were no significant differences in the relative healing rates and PUSH scores between the subpopulations. The median time to complete healing in the study and control populations was 170.0 days (95% confidence interval [CI], 85.7–254.3) and 218.0 days (95% CI, 149.2–286.7) (log-rank, chi-square=3.99; p<0.046), respectively.
-
Conclusion
- More than 20 weeks of HMB/Arg/Gln supplementation had a positive effect on difficult PU healing in older adults with multiple comorbidities.
- Beta-hydroxy-beta-methylbutyrate (HMB) is a metabolite of the essential amino acid, leucine. Its consumption promotes the building and strengthening of muscle tissue and increases fat oxidation. Since the beginning of the current millennium, its consumption has grown mainly among sportsmen and women [1]. The working hypothesis in studies conducted during this period was that HMB is an active ingredient in the anticatabolic effects of leucine and other metabolites.
- The combination of HMB with the amino acids arginine (Arg) and glutamine (Gln) is intended for the treatment of patients requiring support in building lean body mass [2]. HMB/Arg/Gln is given as a supplement in the daily nutritional regimen for the healing of recalcitrant wounds. Examples of such wounds include pressure ulcers (PUs) in patients in general hospitals [3], wounds experienced by patients who are diabetic [4-6], burn injuries [7], and patients in intensive care units [8].
- The combination of HMB/Arg/Gln has been shown in studies to assist in the production of collagen [9], building of protein and muscle, and improvement in lean body mass, including in healthy individuals who are older [10]. Thus, HMB/Arg/Gln can assist in wound healing. In addition, HMB/Arg/Gln supports immune functions and is especially important in healing and recovery processes [11,12]. A summary of the studies that have demonstrated the effect of HMB/Arg/Gln on wound healing is shown in Table 1. From these few studies, it is clear that the healing of these types of wounds and injuries can benefit from HMB/Arg/Gln administration.
- HMB might limit tissue damage among patients who are older [13] and those who are confined to their beds [14], and it may even prevent atrophy of muscle tissue [15]. Recently, HMB has also been used in individuals who are older to preserve and strengthen muscle tissue [16,17]. In contrast to what has been mentioned above, and as can be seen in Table 1, the hypothesized effect of long-term administration of HMB/Arg/Gln in the treatment of PUs among older patients in geriatric and rehabilitation facilities has not yet been scientifically validated. The objective of the current study was to determine the association between long-term oral consumption of HMB/Arg/Gln and healing of PUs among bedridden older patients in a geriatric and rehabilitation facility.
Introduction
- Ethical statements: This study was approved by the Institutional Review Board (IRB) of Bayit Balev Geriatric and Rehabilitation Center (IRB No: 0009-21-BBL). Since the patient care process was not influenced by the current study, informed consent was not required.
- 1. Study design
- This is a pilot retrospective matched case-control clinical study. The participants of the case population received standard of care [18] with HMB/Arg/Gln, and the participants of the control group received only standard of care.
- 2. Setting
- This study was conducted at a geriatric and rehabilitation center. This setting has been described elsewhere [19].
- 3. Participants
- The participants developed PUs during their hospitalization or were admitted with preexisting PUs.
- The inclusion criteria were: (1) both sexes; (2) 65 years or older; (3) hospitalized in Bayit Balev Geriatric and Rehabilitation Center between January 1, 2011 and May 1, 2021; and (4) diagnosed with stage 2, 3, or 4 PUs (new onset, chronic, etc.).
- The exclusion criteria included the following: (1) current treatment with radiation, chemotherapy, immunosuppressive agents, corticosteroids, or dialysis; (2) concurrent active or severe comorbidity that may have interfered with PU healing (e.g., vasculitis, immune system disorder, carcinoma, and connective tissue disease); (3) known current addiction to psychoactive substances; and (4) multiple diabetic ulcers on the same site for patients with diabetes mellitus (DM).
- In the data collection process, the data of participants in the study subpopulation were first included. The matching criteria were demographic (age and sex) and clinical (main diagnosis and baseline area and perimeter of the PU) parameters. Thereafter, the data of the participants in the control subpopulation were collected.
- 4. Intervention
- The participants in the study subpopulation received oral supplementation with HMB/Arg/Gln (orally or via feeding tube) twice per day until PU healing or closure. The 24-g bags of supplement contained 1.3-g calcium HMB, 7.4-g ʟ-Arg, and 7.4-g ʟ-Gln (Abound; Abbott Laboratories, West Chicago, IL, USA) in 250 mL of water (89 kcal total energy).
- 5. Outcome measures
- The reduction in absolute area (current area−baseline area), percentage change in area (100×[current area−baseline area/baseline area]), and linear advancement from the wound edge ([current area−baseline area]/[current perimeter+baseline perimeter]/2) [20] were calculated after 4, 8, 12, 16, and 20 weeks.
- A valid, sensitive, and simple instrument to monitor the healing of stage 2 to 4 PUs [3], including in older populations, is Pressure Ulcer Scale for Healing (PUSH) tool, ver. 3.0. It consists of three parameters: length×width, exudate amount (heavy, moderate, light, and none), and tissue type (necrotic tissue, slough, granulation tissue, epithelial tissue, and closed). Each parameter is scored, and the sum of the three yields a total wound status score, where 0=completely healed and 17=worst possible score, indicating the greatest severity. Observation of the changes in the direction and magnitude of the score over time indicates whether wound healing is occurring. Because PUSH involves only three parameters, it is easy to use and takes less than 1 minute to complete [21,22]. The PUSH scores were calculated after 4, 8, 12, 16, and 20 weeks.
- The time to complete PU healing was measured as the number of days from the initiation of treatment to the date that a participant achieved complete PU healing or closure [20,23], regardless of the time required.
- 6. Baseline assessment
- All participants were evaluated as an integrated part of their care during the clinical assessment process. Baseline PU length and width were measured before beginning the treatment process. The rectangular area (length×width), perimeter ([length+width]×2) [24], elliptical area (length×width×π/4) [24,25], and shape (length×width×0.73) [26] were calculated.
- In the current study, the Charlson comorbidity index (CCI) was used to classify comorbid conditions. Using this index, it is possible to predict the mortality risk with multiple comorbid conditions. The index does not consider past conditions (e.g., past pneumonia) or past surgeries for conditions that are no longer active (e.g., removal of the gall bladder or appendix). However, all chronic and active conditions, rare and common, are considered by this index (by means of the measurement “existing” or “not existing”). Each condition is assigned its own weight. The higher the general score, the more pervasive is the accompanying illness. A score is also assigned to age; for every decade after 40 years of age, one point is assigned [27].
- The staging system classifies PUs into four stages (stages 1–4) according to the dimensions of the damaged region of the tissue. However, the numerical staging does not always indicate linear progression of PUs. For example, a small lesion may represent substantial necrosis and vice versa. Similarly, the scale does not imply that healing proceeds from stage 4 through stage 1 [28].
- 7. Follow-up and final assessment
- The data, which were collected as part of the standard treatment protocol in general and the treatment protocol for PUs [18] in particular, were processed retrospectively. The data were collected from the case files of the hospitalized patients. All participants were monitored as an integral part of their standard of care in the clinical treatment process. Follow-up measurements were completed weekly and at the end of clinical treatment.
- For the results presented in the current study, the data were summarized every 4 weeks (4, 8, 12, 16, and 20 weeks for the primary endpoints). All measures were based on ruler-based techniques. The date on which a participant achieved complete PU healing or closure (regardless of the treatment time and/or study period) was defined as the secondary endpoint.
- Blood test results in the current study included albumin and hemoglobin levels.
- 8. Statistical methods
- Data were analyzed using IBM SPSS ver. 20.0 for Windows (IBM Corp., Armonk, NY, USA). Continuous variables are expressed as medians with interquartile range (IQR) or 95% confidence interval (CI). Categorical variables are expressed as frequencies and percentages. Demographic and clinical characteristics were compared between the subgroups using the Mann-Whitney U-test or chi-square test, as appropriate. The change from baseline of blood albumin and hemoglobin levels in each subpopulation was compared using the Wilcoxon signed-rank test. Repeated-measures analysis of variance was conducted for PUSH scores.
- The between-groups analysis of time to healing was presented in the following steps: incidence of healed PUs over time using Kaplan-Meier survival estimates (with log-rank test) and a Cox proportional hazards model of time to healing, adjusted for any influential factors. Hazard ratios (HRs) and 95% CIs were used to assess the risk of PU healing. All p-values were two-sided, and a p-value of less than 0.05 was considered to indicate statistical significance.
Methods
1) Relative healing rates
2) Pressure Ulcer Scale for Healing
3) Time to complete healing
- 1. Descriptive data of participants
- The study sample was comprised of 45 participants. A description of the study population and between-group comparisons are presented in Table 2. From Table 2, it can be seen that there were no significant differences between the study group and control group in terms of age, sex, main diagnosis, prevalence of participants who had been on prolonged mechanical ventilation (PMV), having DM or dementia, PU site, baseline area and perimeter of PU, PUSH scores, and blood level of albumin (not significant [NS], for all).
- However, the proportion of participants with stage 4 PUs was much greater (p<0.003) and the level of serum hemoglobin was lower (p<0.006) in the study group than in the control group. In addition, there was a trend toward greater comorbidities in the study group than in the control group (p<0.06).
- 2. Outcome data
- After follow-up at 4, 8, 12, 16, and 20 weeks, no significant differences were found between the study and control groups with respect to the absolute area of the wound, percentage reduction in the wound area (NS, for everyone), and linear progression of the boundary of the wound (NS, for everyone) (Table 3).
- Repeated-measures analysis of variance was conducted. Mauchly’s sphericity test was significant (Mauchly’s W=0.05; degree of freedom [df]=14; p<0.0001); therefore, we accepted that the variances of the differences between PUSH score levels were significantly different. Therefore, the condition of sphericity was not met, even after Huynh-Feldt and Greenhouse-Geisser corrections (F=37.493; p<0.0001, for both corrections).
- Using the Mann-Whitney U-test, statistically significant differences in PUSH scores between the subpopulations were not found (NS, for everyone) (Table 3).
- A statistically significant difference was found in the amount of time that was required for complete wound healing. In the study population, the median time was 170.0 days (95% CI, 85.7–254.3 days); in the control population, the median time was 218.0 days (95% CI, 149.2–286.7 days) (log-rank, chi-square=3.99; df, 1; p<0.046).
- 3. Predicting complete healing
- A trend in the statistically significant prediction of complete healing was identified. Specifically, the addition of HMB/Arg/Gln to the treatment regimen for PUs increased the HR for healing (HR, 2.46; 95% CI, 1.00–6.12; p<0.053) (Table 4, Fig. 1).
- 4. Adverse events
- There were no adverse events during the study period.
Results
1) Relative healing rates
2) Pressure Ulcer Scale for Healing score
3) Time to complete healing
- This unique study was intended to investigate the association between the long-term oral intake of HMB/Arg/Gln and healing of PUs among bedridden older patients with significant comorbidities in a geriatric and rehabilitation facility. Relative healing rates, PUSH scores, and time to healing parameters were assessed. Among the key findings in this study, the addition of HMB/Arg/Gln to the regular standard of care regimen in the treatment of PUs among bedridden older patients does not improve relative healing rates (as measured by the reduction in absolute area, percentage change in area, and linear advancement from the wound edge) or PU healing (as measured by the PUSH Tool, ver. 3). We could not find similar studies in this area examining older patients, but our findings support those in studies on the treatment of PUs among younger subjects [3], in research studies with a follow-up period of up to 4 weeks [3,4,8] or 16 weeks [5], and even in studies on older patients with fewer comorbidities [13].
- However, the addition of HMB/Arg/Gln to the standard of care for PUs among bedridden patients who are older significantly shortened the healing time in comparison to regular standard of care without HMB/Arg/Gln treatment. This key finding demonstrates the significant advantage of adding HMB/Arg/Gln to the standard of care for PUs among older patients.
- Among the possible explanations for the additional benefit provided by HMB/Arg/Gln in the healing of PUs in the current study are improved nitrogen balance, not necessarily due to a reduction in the rate of protein metabolism [29]; promotion of collagen production [9], including among patients who are diabetic [6]; and improvements in hematological parameters [30], protein balance [7], vascular endothelial function [12], and inflammation [31].
- It must be emphasized that, at the start of the follow-up period, no differences were detected between the study subgroups with respect to matching indices (age, sex, main diagnoses, baseline area, and PU perimeter) or other indices (e.g., prevalence of participants who had been on PMV and DM prevalence). However, the participants in the study group were more complicated with respect to additional measures (CCI, PU stage, and blood hemoglobin level), which became clearer during the data analysis stage of the study (Table 2). We are certain that all these measures made a significant contribution to the finding of an absence of differences in the relative healing rates between the study subgroups and lengthening of the time to healing of PUs.
- In addition, what cannot be ignored is the fact that, during follow-up, the increase in blood hemoglobin level was higher in the study group (median change from baseline, 0.75 g/dL; p<0.07) than in the control group (median change from baseline, 0.4 g/dL; p<0.05). This was in accordance with an earlier study in this area in which an improvement in hematological measures in general and an increase in blood hemoglobin levels in particular were observed as a result of the addition of HMB/Arg/Gln treatment among healthy adult males, patients with AIDS-associated weight loss, and patients with cancer who were experiencing wasting [28].
- The importance of this pilot study is that it describes the association between long-term nutritional interventions with HMB/Arg/Gln and PU healing, and the resulting health benefits for sedentary older adults with significant comorbidities in geriatric and rehabilitation facilities.
- Simple ruler methods (rectangular area) are easy to use and are inexpensive, but they overestimate the wound area. Mathematical models (elliptical area and shape measurement) are fast, easy to use, and noninvasive, but are inaccurate when assessing wounds with irregular shapes. In our study, these methods were used for between-population comparisons only, and not for the estimation of the treatment effect on wound area.
- Currently, no simple, valid, and reliable technique for measuring wound volume is available. The overall clinical benefit of measuring wound volume has not been established; therefore, it cannot be recommended [20,24,26]. As a result, this measurement was not performed in the current study.
- It is possible that mostly complicated cases were included in the current study, namely older patients with multiple comorbidities and complicated PUs that required treatment intervention for a prolonged period of 6 months or longer. In previous studies, the follow-up period was considerably shorter (4 weeks) in one retrospective study [4] and up to 16 weeks in randomized controlled trials [3,5,13]. However, with the prolongation of life expectancy and current improvements in medical technologies, there is a possibility that our sample population is representative of a real-world population of older adults today and in the near future.
- In conclusion, the long-term addition of HMB/Arg/Gln to the standard treatment regimen for PUs among bedridden older patients with substantial comorbidities in geriatric and rehabilitation facilities significantly shortens the healing time of PUs in comparison to the standard of care regimen without HMB/Arg/Gln. It is recommended that a controlled clinical study (double-blind) be conducted to obtain reliable empirical evidence of the assumed impact of the long-term addition of HMB/Arg/Gln in the treatment of PUs among the bedridden older population.
Discussion
-
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
-
Funding
None.
-
Author contributions
Conceptualization, Data curation: IK, YG; Formal analysis, Software, Supervision, Validation: YG; Project administration, Resources: IK; Writing-original draft: YG; Writing-review & editing: YG.
Notes
| Study | Type | Study population | Age (yr) | Male sex (%) | Dosage | Treatment duration (wk) | Assessment | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Sipahi et al. [4] | Retrospective | Diabetic hemodialysis patients (n=11) | Mean, 66.0 (SD, 10.0) | 81.8 | * | 4.0 | BWAT | Positive effect |
| Wong et al. [3] | RCT | Patients with PU in general hospital (n=11)a) | ≥21 | ** | 2.0 | PUSH | No change | |
| Viable tissue | Increased | |||||||
| Wound area | No change | |||||||
| Armstrong et al. [5] | RCT | Patients with diabetic foot | Median, 58.0 (range, 28–86)a) | 72.1 | ** | 16.0 | Wound closure | No difference |
| ulcers (n=105)a) | Time to healing | No difference | ||||||
| Dennis et al. [11] | RCT | Vascular endothelial function in older adults (n=16)a) | Mean, 72.6 (SD, 6.0) | 43.8 | *** | 24.0 | Flow-mediated dilation of the brachial artery | 27% increase |
| C-reactive protein | No change | |||||||
| Tumor necrosis factor-α | No change | |||||||
| Miu [13] | RCT | PU in older adults (n=28)a) | Mean, 83.04 (SD, 11.5)a) | 61.7 | * | 4.0 | PU size/depth/undermine | No change |
| PUSH | No change | |||||||
| Length of hospitalization | No change | |||||||
| Number of readmissions | No change | |||||||
| Mortality | No change | |||||||
| Biochemical parameters | No change | |||||||
| Current study | Retrospective | PU in sedentary older adults in long-term care units (n=14)a) | Median, 85.5 (IQR, 82.0–90.2)a) | 28.6 | * | 20.0 | Relative healing rates | No difference |
| 20.0 | PUSH | No change | ||||||
| No limit | Time to healing | Positive effect |
HMB, beta-hydroxy-beta-methylbutyrate; Arg, arginine; Gln, glutamine; SD, standard deviation; BWAT, Bates-Jensen Wound Assessment Tool; RCT, randomized controlled trial; PU, pressure ulcers; PUSH, Pressure Ulcer Scale for Healing tool, ver. 3; IQR, interquartile range.
Dosage: *1.3-g HMB, 7.4-g Arg, 7.4-g Gln×2/day; **1.2-g HMB, 7.0-g Arg, 7.0-g Gln×2/day; ***1.5-g HMB, 7.0-g Arg, 7.0-g Gln×2/day.
a) Only for study population with HMB/Arg/Gln.
Values are presented as median (interquartile range) or number (%).
HMB, beta-hydroxy-beta-methylbutyrate; Arg, arginine; Gln, glutamine; PMV, prolonged mechanical ventilation; DM, diabetes mellitus; CCI, Charlson comorbidity index; PU, pressure ulcer; PUSH, Pressure Ulcer Scale for Healing tool, ver. 3.
| Variable | Standard of care+HMB/Arg/Gln (n=14) | Standard of care (n=31) | p-value |
|---|---|---|---|
| PUSH scorea) | |||
| Baseline | 13.5 (12.5–15.0) | 13.0 (11.0–14.0) | 0.21 |
| After 4 weeks | |||
| Absolute area reduction | 0.0 (–9.1–13.5) | 0.0 (–15.0–2.0) | 0.41 |
| Percentage reduction in area | 0.0 (–34.6–10.8) | 0.0 (–64.0–45.8) | 0.50 |
| Linear advancement of PU edge | 0.0 (–0.6–0.3) | 0.0 (–0.9–0.2) | 0.42 |
| PUSH score | 14.0 (11.5–14.2) | 13.0 (10.0–14.0) | 0.20 |
| After 8 weeks | |||
| Absolute area reduction | –14.8 (–19.4–6.8) | –5.2 (–27.2–1.8) | 0.96 |
| Percentage reduction in area | –43.2 (–74.1–25.3) | –29.4 (–90.6–37.5) | 0.69 |
| Linear advancement of PU edge | –0.6 (–1.2–0.3) | –0.5 (–1.8–0.2) | 0.74 |
| PUSH score | 12.0 (10.0–13.0) | 11.0 (9.0–13.0) | 0.46 |
| After 12 weeks | |||
| Absolute area reduction | –19.1 (–23.6–6.0) | –7.0 (–33.2–0.0) | 0.40 |
| Percentage reduction in area | –78.6 (–96.4–44.1) | –46.4 (–99.6–0.0) | 0.54 |
| Linear advancement of PU edge | –1.0 (–1.6–0.7) | –0.4 (–2.5–0.0) | 0.55 |
| PUSH score | 9.5 (2.0–12.0) | 9.0 (0.0–12.0) | 0.86 |
| After 16 weeks | |||
| Absolute area reduction | –21.0 (–27.2–3.0) | –4.8 (–23.8–0.0) | 0.40 |
| Percentage reduction in area | –86.4 (–100.0–64.5) | –60.2 (–96.3–0.0) | 0.22 |
| Linear advancement of PU edge | –1.3 (–2.1–0.8) | –0.6 (–2.0–0.0) | 0.36 |
| PUSH score | 7.0 (0.0–11.0) | 6.0 (0.0–12.0) | 0.87 |
| After 20 weeks | |||
| Absolute area reduction | –24.0 (–36.1–3.8) | –4.9 (–15.4–1.8) | 0.11 |
| Percentage reduction in area | –98.2 (–100.0–83.8) | –62.4 (–97.0–26.9) | 0.08 |
| Linear advancement of PU edge | –1.8 (–2.3–0.9) | –0.6 (–1.7–0.1) | 0.10 |
| PUSH score | 2.5 (0.0–10.0) | 0.0 (0.0–10.2) | 0.81 |
| Follow-up outcomeb) | |||
| Complete healing | 7 (50.0) | 29 (93.5) | |
| Discharge | 6 (42.9) | 1 (3.2) | |
| End of study follow-up | 1 (7.1) | 1 (3.2) | |
| Time to complete healing (day)c) | 170.0 (85.7–254.3) | 218.0 (149.2–286.7) | 0.046 |
| Blood level at the end of follow-upa) | |||
| Albumin (g/L) | 3.1 (2.9–3.3) | 3.2 (2.9–3.4) | 0.74 |
| Hemoglobin (g/dL) | 9.2 (8.1–10.1) | 10.5 (9.9–11.6) | 0.003 |
- 1. Slater GJ, Jenkins D. Beta-hydroxy-beta-methylbutyrate (HMB) supplementation and the promotion of muscle growth and strength. Sports Med 2000;30:105–16.ArticlePubMed
- 2. May PE, Barber A, D’Olimpio JT, Hourihane A, Abumrad NN. Reversal of cancer-related wasting using oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine, and glutamine. Am J Surg 2002;183:471–9.ArticlePubMed
- 3. Wong A, Chew A, Wang CM, Ong L, Zhang SH, Young S. The use of a specialized amino acid mixture for pressure ulcers: a placebo-controlled trial. J Wound Care 2014;23:259𠄰60, 262𠄰4, 266𠄰9.ArticlePubMed
- 4. Sipahi S, Gungor O, Gunduz M, Cilci M, Demirci MC, Tamer A. The effect of oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine and glutamine on wound healing: a retrospective analysis of diabetic haemodialysis patients. BMC Nephrol 2013;14:8.ArticlePubMedPMCPDF
- 5. Armstrong DG, Hanft JR, Driver VR, Smith AP, Lazaro-Martinez JL, Reyzelman AM, et al. Effect of oral nutritional supplementation on wound healing in diabetic foot ulcers: a prospective randomized controlled trial. Diabet Med 2014;31:1069–77.ArticlePubMedPMCPDF
- 6. Jones MS, Rivera M, Puccinelli CL, Wang MY, Williams SJ, Barber AE. Targeted amino acid supplementation in diabetic foot wounds: pilot data and a review of the literature. Surg Infect (Larchmt) 2014;15:708–12.ArticlePubMedPMC
- 7. Erdem D, Sözen İ, Çakırca M, Örnek D, Kanyılmaz D, Akan B. Effect of nutritional support containing arginine, glutamine and β-hydroxy-β-methylbutyrate on the protein balance in patients with major burns. Turk J Anaesthesiol Reanim 2019;47:327–33.ArticlePubMedPMC
- 8. Erdem D, Akan B, Albayrak MD, Aksoy E, Unal S, Ornek D, et al. The use of nutritional supplement containing arginine, glutamine and beta-hydroxy-beta-methylbutyrate in the treatment of pressure ulcers in an ICU patient. Analg Resusc Curr Res 2015;4:1.Article
- 9. Williams JZ, Abumrad N, Barbul A. Effect of a specialized amino acid mixture on human collagen deposition. Ann Surg 2002;236:369–75.ArticlePubMedPMC
- 10. Ellis AC, Hunter GR, Goss AM, Gower BA. Oral supplementation with beta-hydroxy-beta-methylbutyrate, arginine, and glutamine improves lean body mass in healthy older adults. J Diet Suppl 2019;16:281–93.ArticlePubMed
- 11. Dennis RA, Ponnappan U, Kodell RL, Garner KK, Parkes CM, Bopp MM, et al. Immune function and muscle adaptations to resistance exercise in older adults: study protocol for a randomized controlled trial of a nutritional supplement. Trials 2015;16:121.ArticlePubMedPMCPDF
- 12. Ellis AC, Patterson M, Dudenbostel T, Calhoun D, Gower B. Effects of 6-month supplementation with β-hydroxy-β-methylbutyrate, glutamine and arginine on vascular endothelial function of older adults. Eur J Clin Nutr 2016;70:269–73.ArticlePubMedPDF
- 13. Miu KY. The use of an oral mixture of arginine, glutamine and β-hydroxy-β-methylbutyrate (Hmb) for the treatment of high grade pressure ulcers: a randomized study. Aging Med Healthc 2021;12:82–9.Article
- 14. Hsieh LC, Chow CJ, Chang WC, Liu TH, Chang CK. Effect of beta-hydroxy-beta-methylbutyrate on protein metabolism in bed-ridden elderly receiving tube feeding. Asia Pac J Clin Nutr 2010;19:200–8.PubMed
- 15. Wu H, Xia Y, Jiang J, Du H, Guo X, Liu X, et al. Effect of beta-hydroxy-beta-methylbutyrate supplementation on muscle loss in older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr 2015;61:168–75.ArticlePubMed
- 16. Bear DE, Langan A, Dimidi E, Wandrag L, Harridge SD, Hart N, et al. β-Hydroxy-β-methylbutyrate and its impact on skeletal muscle mass and physical function in clinical practice: a systematic review and meta-analysis. Am J Clin Nutr 2019;109:1119–32.ArticlePubMed
- 17. Costa Riela NA, Alvim Guimarães MM, Oliveira de Almeida D, Araujo EM. Effects of beta-hydroxy-beta-methylbutyrate supplementation on elderly body composition and muscle strength: a review of clinical trials. Ann Nutr Metab 2021;77:16–22.ArticlePubMedPDF
- 18. Gould L, Stuntz M, Giovannelli M, Ahmad A, Aslam R, Mullen-Fortino M, et al. Wound Healing Society 2015 update on guidelines for pressure ulcers. Wound Repair Regen 2016;24:145–62.ArticlePubMed
- 19. Leonov Y, Kisil I, Perlov A, Stoichev V, Ginzburg Y, Nazarenko A, et al. Predictors of successful weaning in patients requiring extremely prolonged mechanical ventilation. Adv Respir Med 2020;88:477–84.ArticlePubMed
- 20. Jessup RL. What is the best method for assessing the rate of wound healing?: a comparison of 3 mathematical formulas. Adv Skin Wound Care 2006;19:138–47.ArticlePubMed
- 21. Stotts NA, Rodeheaver GT, Thomas DR, Frantz RA, Bartolucci AA, Sussman C, et al. An instrument to measure healing in pressure ulcers: development and validation of the pressure ulcer scale for healing (PUSH). J Gerontol A Biol Sci Med Sci 2001;56:M795–9.ArticlePubMed
- 22. Gardner SE, Frantz RA, Bergquist S, Shin CD. A prospective study of the pressure ulcer scale for healing (PUSH). J Gerontol A Biol Sci Med Sci 2005;60:93–7.ArticlePubMed
- 23. Veves A, Sheehan P, Pham HT. A randomized, controlled trial of Promogran (a collagen/oxidized regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcers. Arch Surg 2002;137:822–7.ArticlePubMed
- 24. Keast DH, Bowering CK, Evans AW, Mackean GL, Burrows C, D’Souza L. MEASURE: a proposed assessment framework for developing best practice recommendations for wound assessment. Wound Repair Regen 2004;12(3 Suppl):S1–17.ArticlePubMed
- 25. Kantor J, Margolis DJ. Efficacy and prognostic value of simple wound measurements. Arch Dermatol 1998;134:1571–4.ArticlePubMed
- 26. Jørgensen LB, Sørensen JA, Jemec GB, Yderstraede KB. Methods to assess area and volume of wounds: a systematic review. Int Wound J 2016;13:540–53.ArticlePubMed
- 27. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83.ArticlePubMed
- 28. Grada A, Phillips TJ. Pressure injuries [Internet]. In: Editorial staff of Merck & Co., Inc. Merck Manual for the Professional Rahway (NJ): Merck & Co., Inc.; 2022 [cited 2022 Nov 21]. https://www.msdmanuals.com/professional/dermatologic-disorders/pressure-injury/pressure-injuries#.
- 29. Kuhls DA, Rathmacher JA, Musngi MD, Frisch DA, Nielson J, Barber A, et al. Beta-hydroxy-beta-methylbutyrate supplementation in critically ill trauma patients. J Trauma 2007;62:125–32.ArticlePubMed
- 30. Rathmacher JA, Nissen S, Panton L, Clark RH, Eubanks May P, Barber AE, et al. Supplementation with a combination of beta-hydroxy-beta-methylbutyrate (HMB), arginine, and glutamine is safe and could improve hematological parameters. JPEN J Parenter Enteral Nutr 2004;28:65–75.ArticlePubMed
- 31. Gündğdu RH, Temel H, Bozkırlı BO, Ersoy E, Yazgan A, Yıldırım Z. Mixture of arginine, glutamine, and β-hydroxy-β-methyl butyrate enhances the healing of ischemic wounds in rats. JPEN J Parenter Enteral Nutr 2017;41:1045–50.ArticlePubMedPDF
References
Figure & Data
References
Citations

- Impact of oral nutritional supplement composition on healing of different chronic wounds: A systematic review
Allan Carlos Soares do Espírito Santo, Clara Sandra de Araújo Sugizaki, Alcides Corrêa de Morais Junior, Nara Aline Costa, Maria Marcia Bachion, João Felipe Mota
Nutrition.2024; 124: 112449. CrossRef

 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine PubReader
PubReader ePub Link
ePub Link Cite
Cite